
Am Fam Physician. 2016;93(8):682-684
Author disclosure: No relevant financial affiliations.
Ivabradine (Corlanor) is labeled for the reduction of hospitalizations in patients with chronic systolic heart failure. It inhibits the so-called funny current within the sinoatrial node, reducing heart rate without lowering blood pressure.1 Ivabradine is added to preexisting maximal medical treatment.
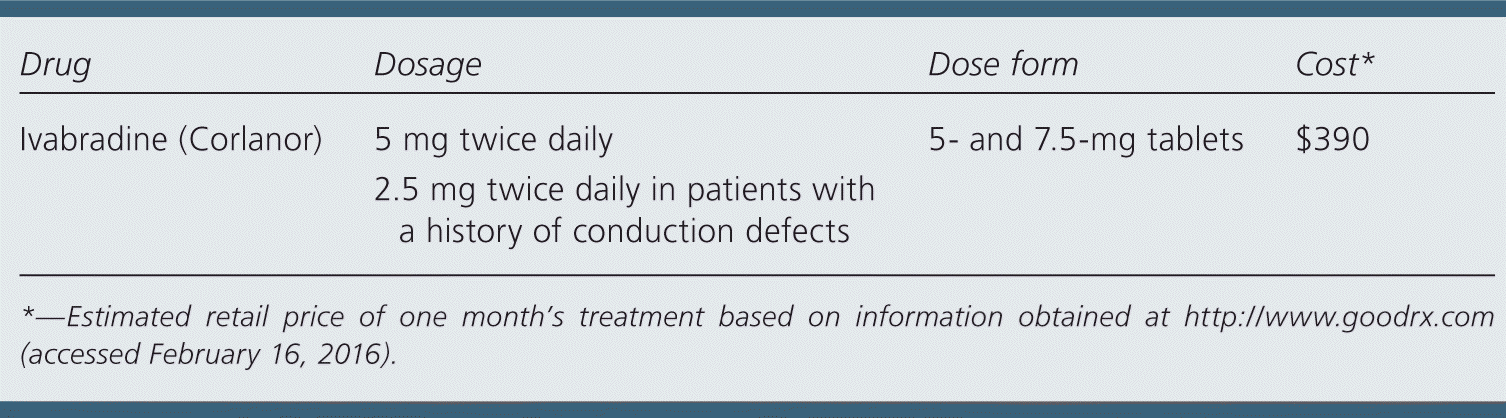
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Ivabradine (Corlanor) | 5 mg twice daily | 5- and 7.5-mg tablets | $390 |
| 2.5 mg twice daily in patients with a history of conduction defects |
SAFETY
Atrial fibrillation, bradycardia, and hypertension may occur in patients taking ivabradine. As demonstrated in clinical trials, 5.3% to 9% of patients will develop atrial fibrillation vs. 3.8% to 8% of patients treated with placebo (number needed to treat to harm = 55 to 100). Bradycardia will occur in 10% to 13% of patients, most notably in those with cardiac conduction disorders, a low resting heart rate, and in patients also taking digoxin, diltiazem, verapamil, or amiodarone. Conduction disturbances such as sinus arrest and heart block may also occur.2,3
Ivabradine is contraindicated in patients with severe hepatic impairment, sick sinus syndrome, sinoatrial block, second- or third-degree atrioventricular block (unless a functioning demand pacemaker is present), a resting heart rate less than 60 beats per minute (bpm), or pacemaker dependence.3
Ivabradine is metabolized by the cytochrome P450 (CYP450) system. Verapamil, diltiazem, macrolide antibiotics, protease inhibitors, and other moderate to strong CYP3A4 inhibitors may potentiate its effect and should therefore be avoided. Rifampin, phenytoin (Dilantin), and other inducers of CYP3A4 may reduce its effect.3 Ivabradine is a U.S. Food and Drug Administration pregnancy category D drug.
TOLERABILITY
Approximately one in six patients will discontinue ivabradine due to adverse effects, with 1% of discontinuations attributed to bradycardia.2 On average, ivabradine reduces heart rate by 11 bpm. At the beginning of treatment, patients may experience luminous phenomena (phosphenes) caused by a direct effect of the drug on the retina, described as colored bright lights, image decomposition, halos, and enhanced brightness. These disturbances often resolve during treatment or shortly after discontinuation.3
EFFECTIVENESS
Ivabradine was evaluated in a single study of 6,505 patients with stable New York Heart Association class II to class IV systolic heart failure. These patients had been hospitalized at least once for heart failure in the previous 12 months and were already receiving optimal therapy, including maximally tolerated dosages of beta blockers, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), spironolactone, and diuretics. The addition of ivabradine significantly reduces the combined outcome of hospitalizations from heart failure and cardiovascular death (24% vs. 29% for placebo; number needed to treat [NNT] for two years = 26; 95% confidence interval, 18.5 to 47.3).2 Specifically, ivabradine significantly reduces the rate of hospitalizations due to heart failure by five percentage points from a baseline of 21% (NNT for two years = 20). However, overall cardiovascular mortality is not significantly decreased. It may be associated with a reduction in heart failure–related mortality.2,4 Ivabradine has not been studied in patients with heart failure who have preserved ejection fraction.
PRICE
A one-month supply of ivabradine costs approximately $390. This is in addition to the cost of maximally tolerated therapy with a beta blocker, ACE inhibitor or ARB, aldosterone antagonist, and diuretic.
SIMPLICITY
Before starting ivabradine, patients should be on maximally tolerated dosages of a beta blocker, an ACE inhibitor or ARB, and an aldosterone antagonist.5 They should have a resting heart rate greater than 70 bpm and stable symptoms. The starting dosage is 5 mg twice daily. After two weeks the dosage can be increased to a maximum of 7.5 mg twice daily in patients with a heart rate greater than 60 bpm. The dosage should be lowered to 2.5 mg twice daily in patients with a heart rate less than 50 bpm and symptoms of bradycardia. Patients with conduction abnormalities should be initiated on a reduced dosage of 2.5 mg twice daily.
Bottom Line
In patients with stable systolic heart failure already on maximal medication therapy, adding ivabradine may reduce the number of hospitalizations related to heart failure (NNT for two years = 20), but it does not reduce cardiovascular mortality. In addition, many patients will not tolerate the drug and will stop taking it. Patients should be monitored for the development of atrial fibrillation and bradycardia.
