
Am Fam Physician. 2016;93(10):online
Related Putting Prevention into Practice: Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women.
Summary of Recommendations and Evidence
The USPSTF recommends that clinicians ask all adults about tobacco use, advise them to stop using tobacco, and provide behavioral interventions and U.S. Food and Drug Administration (FDA)–approved pharmacotherapy for cessation to adults who use tobacco (Table 1). A recommendation.
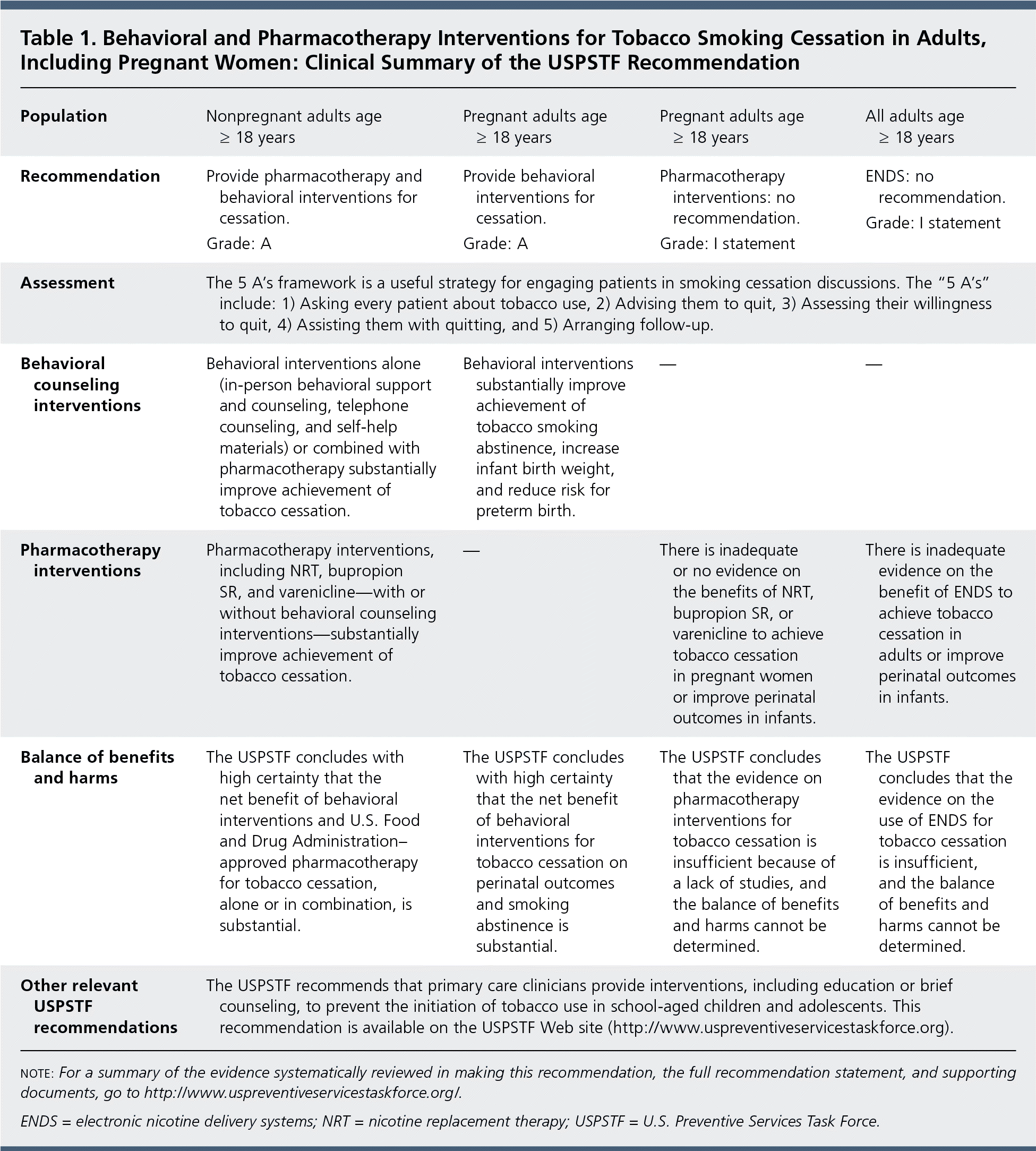
| Population | Nonpregnant adults age ≥ 18 years | Pregnant adults age ≥ 18 years | Pregnant adults age ≥ 18 years | All adults age ≥ 18 years |
| Recommendation | Provide pharmacotherapy and behavioral interventions for cessation. | Provide behavioral interventions for cessation. | Pharmacotherapy interventions: no recommendation. | ENDS: no recommendation. |
| Grade: A | Grade: A | Grade: I statement | Grade: I statement | |
| Assessment | The 5 A's framework is a useful strategy for engaging patients in smoking cessation discussions. The “5 A's” include: 1) Asking every patient about tobacco use, 2) Advising them to quit, 3) Assessing their willingness to quit, 4) Assisting them with quitting, and 5) Arranging follow-up. | |||
| Behavioral counseling interventions | Behavioral interventions alone (in-person behavioral support and counseling, telephone counseling, and self-help materials) or combined with pharmacotherapy substantially improve achievement of tobacco cessation. | Behavioral interventions substantially improve achievement of tobacco smoking abstinence, increase infant birth weight, and reduce risk for preterm birth. | — | — |
| Pharmacotherapy interventions | Pharmacotherapy interventions, including NRT, bupropion SR, and varenicline—with or without behavioral counseling interventions—substantially improve achievement of tobacco cessation. | — | There is inadequate or no evidence on the benefits of NRT, bupropion SR, or varenicline to achieve tobacco cessation in pregnant women or improve perinatal outcomes in infants. | There is inadequate evidence on the benefit of ENDS to achieve tobacco cessation in adults or improve perinatal outcomes in infants. |
| Balance of benefits and harms | The USPSTF concludes with high certainty that the net benefit of behavioral interventions and U.S. Food and Drug Administration–approved pharmacotherapy for tobacco cessation, alone or in combination, is substantial. | The USPSTF concludes with high certainty that the net benefit of behavioral interventions for tobacco cessation on perinatal outcomes and smoking abstinence is substantial. | The USPSTF concludes that the evidence on pharmacotherapy interventions for tobacco cessation is insufficient because of a lack of studies, and the balance of benefits and harms cannot be determined. | The USPSTF concludes that the evidence on the use of ENDS for tobacco cessation is insufficient, and the balance of benefits and harms cannot be determined. |
| Other relevant USPSTF recommendations | The USPSTF recommends that primary care clinicians provide interventions, including education or brief counseling, to prevent the initiation of tobacco use in school-aged children and adolescents. This recommendation is available on the USPSTF Web site (http://www.uspreventiveservicestaskforce.org). | |||
The USPSTF recommends that clinicians ask all pregnant women about tobacco use, advise them to stop using tobacco, and provide behavioral interventions for cessation to pregnant women who use tobacco. A recommendation.
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of pharmacotherapy interventions for tobacco cessation in pregnant women. I statement.
The USPSTF concludes that the current evidence is insufficient to recommend electronic nicotine delivery systems (ENDS) for tobacco cessation in adults, including pregnant women. The USPSTF recommends that clinicians direct patients who smoke tobacco to other cessation interventions with established effectiveness and safety (previously stated). I statement.
See the Clinical Considerations section for suggestions for practice regarding the I statements.
Rationale
IMPORTANCE
Tobacco use is the leading preventable cause of disease, disability, and death in the United States. Cigarette smoking results in more than 480,000 premature deaths each year and accounts for approximately 1 in every 5 deaths.1 In pregnant women, smoking increases the risk for congenital anomalies; perinatal complications, such as preterm birth, fetal growth restriction, and placental abruption; miscarriage and stillbirth; and neonatal or pediatric complications, such as sudden infant death syndrome and impaired lung function in childhood.1–4 An estimated 42.1 million U.S. adults (nearly 18% of the population) currently smoke.5
RECOGNITION OF BEHAVIOR
The benefits of assessing patients' smoking behavior are well established. Common approaches for clinicians include recording a patient's smoking status as a vital sign or using the 5 A's: 1) Ask about smoking; 2) Advise to quit through clear, personalized messages; 3) Assess willingness to quit; 4) Assist in quitting; and 5) Arrange follow-up and support. Another approach is “Ask, Advise, Refer,” which encourages clinicians to ask patients about tobacco use, advise them to quit, and refer them to telephone quit lines and/or other evidence-based cessation interventions.
BENEFITS OF INTERVENTIONS
Nonpregnant Adults. The USPSTF found convincing evidence that behavioral interventions (including in-person behavioral support and counseling, telephone counseling, and self-help materials) alone or combined with pharmacotherapy substantially improve achievement of tobacco cessation in nonpregnant adults who smoke. The USPSTF found convincing evidence that pharmacotherapy interventions, including nicotine replacement therapy (NRT), bupropion hydrochloride sustained-release (buproprion SR), and varenicline—with or without behavioral counseling interventions—substantially improve achievement of tobacco cessation in nonpregnant adults who smoke. The USPSTF also found convincing evidence that using 2 types of NRT moderately improves achievement of tobacco smoking cessation over using 1 type and that addition of NRT to treatment with bupropion SR provides additional benefit over use of bupropion SR alone. The USPSTF found inadequate evidence to determine the effect of ENDS on achievement of tobacco smoking cessation.
Pregnant Women. The USPSTF found convincing evidence that behavioral interventions substantially improve achievement of tobacco smoking abstinence in pregnant women, increase infant birth weight, and reduce risk for preterm birth. The USPSTF found inadequate evidence on the benefits of NRT and no evidence on the benefits of bupropion SR, varenicline, or ENDS to achieve tobacco cessation in pregnant women who smoke or to improve perinatal outcomes in infants.
HARMS OF INTERVENTIONS
Nonpregnant Adults. The USPSTF determined that there is adequate evidence to bound the magnitude of harms of behavioral interventions for tobacco cessation in nonpregnant adults who smoke as small to none. The USPSTF found adequate evidence that the harms of NRT, bupropion SR, or varenicline for tobacco cessation in adults who smoke are small. The USPSTF found inadequate evidence to determine the harms of ENDS.
Pregnant Women. The USPSTF determined that there is adequate evidence to bound the magnitude of harms of behavioral interventions for tobacco cessation in pregnant women who smoke as small to none. The USPSTF found inadequate evidence on the harms of NRT and no evidence on the harms of bupropion SR, varenicline, or ENDS for tobacco cessation in pregnant women who smoke.
USPSTF ASSESSMENT
The USPSTF concludes with high certainty that the net benefit of behavioral interventions and FDA-approved pharmacotherapy for tobacco cessation, alone or combined, in nonpregnant adults who smoke is substantial.
The USPSTF concludes with high certainty that the net benefit of behavioral interventions for tobacco cessation on perinatal outcomes and smoking abstinence in pregnant women who smoke is substantial.
The USPSTF concludes that the evidence on pharmacotherapy interventions for tobacco cessation in pregnant women is insufficient because of a lack of studies, and the balance of benefits and harms cannot be determined.
The USPSTF concludes that the evidence on the use of ENDS for tobacco smoking cessation in adults, including pregnant women, is insufficient, and the balance of benefits and harms cannot be determined. The USPSTF has identified the lack of well-designed, randomized controlled trials (RCTs) on ENDS that report smoking abstinence or adverse events as a critical gap in the evidence.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to adults aged 18 years or older, including pregnant women. The USPSTF previously issued a separate recommendation statement on primary care interventions for tobacco use in children and adolescents (available at http://www.uspreventiveservicestaskforce.org). Although the USPSTF acknowledges that tobacco may be used in other forms and that other substances aside from tobacco may be smoked, they are not the focus of this recommendation.
ASSESSMENT OF RISK
According to the 2012–2013 National Adult Tobacco Survey, smoking prevalence is higher in the following groups: men; adults aged 25 to 44 years; persons with a race or ethnicity category of “other, non-Hispanic”; persons with a GED (vs. graduate-level education); persons with an annual household income of less than $20,000; and persons who are lesbian, gay, bisexual, or transgender.6 Higher rates of smoking have been found in persons with mental health conditions.7
IMPLEMENTATION CONSIDERATIONS OF BEHAVIORAL AND PHARMACOTHERAPY INTERVENTIONS
Assessment of Smoking Status
The 5 A's framework (available at http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html) is a useful strategy for engaging patients in discussions about smoking cessation. This program includes the following: 1) Asking every patient about tobacco use, 2) Advising all tobacco users to quit, 3) Assessing their willingness to attempt to quit, 4) Assisting with attempts to quit, and 5) Arranging follow-up.10 “Ask, Advise, Refer” is another approach and involves asking patients about tobacco use, advising those who smoke to quit, and referring them to evidence-based interventions. Treating smoking status as a vital sign and recording smoking status at every health visit are also frequently used to assess smoking status. Because many pregnant women who smoke do not report it, using multiple-choice questions to assess smoking status in this group may improve disclosure.9
Nonpregnant Adults
Both intervention types (pharmacotherapy and behavioral interventions) are effective and recommended; combinations of interventions are most effective, and all should be offered. The best and most effective combinations are those that are acceptable to and feasible for an individual patient; clinicians should consider the patient's specific medical history and preferences, and offer and provide the combination that works best for the patient.
Behavioral Interventions
Many behavioral interventions are available to encourage smoking cessation in adults. These interventions can be delivered in the primary care setting or can be referred to community settings with feedback to the primary care provider. Effective behavioral interventions include in-person behavioral support and counseling, telephone counseling, and self-help materials (Table 2). Behavioral interventions may increase rates of smoking abstinence from a baseline range of approximately 5% to 11% in control groups to 7% to 13% in intervention groups.8
| Intensity | |
| Both minimal (< 20 minutes in 1 visit) and intensive (≥ 20 minutes plus > 1 follow-up visit) physician-advice interventions effectively increase the proportion of adults who successfully quit smoking and remain abstinent for ≥ 6 months. | |
| There is a dose–response relationship between the intensity of counseling and cessation rates (i.e., more or longer sessions improve cessation rates). | |
| Duration | |
| Brief, in-person behavioral counseling sessions (< 10 minutes) effectively increase the proportion of adults who successfully quit smoking and remain abstinent for 1 year. | |
| Although less effective than longer interventions, even minimal interventions (< 3 minutes) have been found to increase cessation rates in some studies. | |
| Frequency | |
| Multiple sessions should be provided; according to the Public Health Service guidelines, patients should receive ≥ 4 in-person counseling sessions. | |
| Cessation rates may plateau after 90 minutes of total counseling contact time. | |
| Format | |
| In-person behavioral counseling sessions (individual or group counseling) | |
| Telephone counseling | |
| Tailored, print-based self-help materials | |
| Provider | |
| In-person behavioral counseling sessions: Various types of primary care providers, including physicians, nurses, psychologists, social workers, and cessation counselors | |
| Telephone counseling: Professional counselors or health care providers who are trained to offer advice over the telephone | |
| Content | |
| Assessment of smoking status: | |
| Ask every patient about tobacco use | |
| Advise all tobacco users to quit | |
| Assess willingness of all tobacco users to make an attempt to quit | |
| Assist all tobacco users with their attempt to quit | |
| Arrange follow-up | |
| Effective counseling interventions provide social support and training in practical problem-solving skills: | |
| Training in problem-solving skills includes helping persons who smoke to recognize situations that increase their risk for smoking, develop coping skills to overcome common barriers to quitting, and develop a plan to quit | |
| Basic information about smoking and successful quitting should also be provided | |
| Complementary practices that improve cessation rates include motivational interviewing, assessing readiness to change, and offering more intensive counseling or referrals | |
Both minimal (< 20 minutes in 1 visit) and intensive (≥ 20 minutes plus > 1 follow-up visit) physician-advice interventions effectively increase the proportion of adults who successfully quit smoking and remain abstinent for at least 6 months.8
Brief, in-person behavioral counseling sessions (< 10 minutes) effectively increase the proportion of adults who successfully quit smoking and remain abstinent for 1 year. Although less effective than longer interventions, even minimal interventions (< 3 minutes) have increased cessation rates in some studies.9
○ There is a dose–response relationship between the intensity of counseling and cessation rates (that is, more or longer sessions improve cessation rates).9
– Several sessions should be provided; per the Public Health Service guidelines, patients should receive at least 4 in-person counseling sessions.9
– Cessation rates may plateau after 90 minutes of total counseling contact time.9
○ Effective interventions can be delivered by various types of primary care providers, including physicians, nurses, psychologists, social workers, and cessation counselors.8,9
○ Both individual and group counseling are effective.9
○ Effective counseling interventions provide social support and training in practical problem-solving skills.9
– Training in problem-solving skills includes helping persons who smoke to recognize situations that increase their risk for smoking, develop coping skills to overcome common barriers to quitting, and develop a plan to quit.
– Basic information about smoking and successful quitting should be provided.
– Complementary practices that improve cessation rates include motivational interviewing, assessing readiness to change, and offering more intensive counseling or referrals.9
Telephone counseling interventions are effective.8,9
○ Effective interventions provide at least 3 telephone calls.8
○ Telephone counseling can be provided by professional counselors or health care providers who are trained to offer advice over the telephone.
Providing self-help materials (primarily print-based) that are tailored to the individual patient (that is, beyond a brochure that simply describes the health effects of smoking) is also effective in improving smoking abstinence.8 Evidence on nontailored, print-based, self-help materials; computer-based programs; and mobile phone–based interventions (such as mHEALTH) is mixed, although several trials show promise.8
Pharmacotherapy
The only pharmacotherapy interventions approved by the FDA for the treatment of tobacco dependence in adults are bupropion SR, varenicline, and NRT (including nicotine transdermal patches, lozenges, gum, inhalers, or nasal spray).
Evidence suggests that rates of smoking abstinence may increase from approximately 10% in control groups (placebo or no pharmacotherapy) to 17% in persons using any form of NRT, from roughly 11% in control groups (placebo or no bupropion SR) to 19% in those using bupropion SR, and from approximately 12% in control groups (placebo) to 28% in those using varenicline.8 Information on dosing regimens is available in the package inserts of individual medications or at http://betobaccofree.hhs.gov. Information for consumers on FDA-approved pharmacotherapy for smoking cessation is available at http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm198176.htm.
Combinations of Pharmacotherapy
Using 2 types of NRT has been found to be more effective than using a single type. In particular, there was evidence that combining a nicotine patch with a rapid-delivery form of NRT is more effective than using a single type.
Some studies suggest that NRT in combination with bupropion SR may be more efficacious than bupropion SR alone but not necessarily NRT alone.8
Combinations of Behavioral and Pharmacotherapy Interventions
Combining behavioral and pharmacotherapy interventions may increase cessation rates from approximately 8% to 14% compared with usual care or minimal behavioral interventions (such as self-help materials or brief advice on quitting).
○ These combination interventions often have behavioral components delivered by specialized cessation counselors or trained staff and often use NRT.
○ Combination interventions often involve several sessions (≥ 4) and tend to be more successful with more sessions.
– The largest effect was found in interventions that provided 8 or more sessions, although the difference in effect among the number of sessions was not significant.
– Contact time ranged from 0 to greater than 300 minutes; interventions lasting 91 to 300 minutes were most common.
The addition of behavioral support to pharmacotherapy also significantly increased cessation rates from approximately 18% in persons using pharmacotherapy alone to 21% in those using a combination of pharmacotherapy and behavioral support.
Intensity of behavioral support ranged from 0 to greater than 300 minutes of contact; interventions most often involved greater than 91 minutes of contact (roughly 40% were 91 to 300 minutes, and 60% were > 300 minutes).
Pregnant Women
Behavioral Interventions
Effective behavioral interventions in pregnant women who smoke include counseling, feedback, health education, incentives, and social support. Compared with usual care or controls, behavioral interventions can increase rates of smoking abstinence from approximately 11% to 15% in pregnant women.
Effective behavioral interventions provided more intensive counseling than minimal advice and other standard components of usual care.9
Counseling sessions augmented with messages and self-help materials tailored for pregnant women who smoke increased abstinence rates during pregnancy compared with brief, generic counseling interventions alone.9
○ Counseling specific to pregnant women should include messages about the effects of smoking on both maternal and fetal health and clear, strong advice to quit as soon as possible. Although smoking cessation at any point during pregnancy yields substantial health benefits for the expectant mother and baby, quitting early in pregnancy provides the greatest benefit to the fetus.9
Other Interventions. Health care system–based strategies that have been shown to improve rates of clinical interventions for smoking cessation in primary care settings include implementing an identification system for tobacco users; providing education, resources, and feedback to promote clinician intervention; and dedicating staff to provide treatment for tobacco dependence and assessing the delivery of this treatment in staff performance evaluations.9
USEFUL RESOURCES
Primary care clinicians may find the following resources useful in talking with adults and pregnant women about smoking cessation: Centers for Disease Control and Prevention fact sheets on quitting smoking (http://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/quitting/index.htm), the U.S. Department of Health and Human Services' BeTobaccoFree (http://betobaccofree.hhs.gov/quit-now/index.html#professionals), the U.S. Department of Health and Human Services' Smoke-Free Women (http://women.smokefree.gov/pregnancy-motherhood.aspx), and the Public Health Service's 2008 clinical practice guidelines.9
In addition, the following resources may be useful to primary care clinicians and practices trying to implement interventions for smoking cessation: the Substance Abuse and Mental Health Services Administration–Health Resources and Services Administration Center for Integrated Health Solutions' resources for smoking cessation (http://www.integration.samhsa.gov/health-wellness/wellness-strategies/tobacco-cessation-2), Centers for Disease Control and Prevention state and community resources for tobacco-control programs (http://www.cdc.gov/tobacco/stateandcommunity/index.htm), and the World Health Organization's tool-kit for delivering brief smoking interventions in primary care (http://www.who.int/tobacco/publications/smoking_cessation/9789241506953/en).
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENTS
Pharmacotherapy for Pregnant Women. Although smoking prevalence is lower in pregnant women than nonpregnant women of the same age, approximately 1 in 6 pregnant women aged 15 to 44 years smokes.7 Smoking during pregnancy slows fetal growth, doubles the risk for delivering a baby with low birth weight, and increases the risk for fetal death by 25% to 50%. For women in whom behavioral counseling does not work, other options to promote smoking cessation may be beneficial.
A few studies have evaluated the benefit of NRT on perinatal and child health outcomes. Although results generally suggest a potential benefit, the overall evidence is too limited to draw clear conclusions. NRT is a pregnancy category D medication, which means that there is positive evidence of fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans. However, it has been suggested that NRT may be safer than smoking during pregnancy.4,11 Potential adverse events reported include increased rates of cesarean delivery, slightly increased diastolic blood pressure, and skin reactions to the patch. Potential adverse events reported in nonpregnant adults include higher rates of low-risk cardiovascular events, such as tachycardia. There is no evidence of perinatal harms from NRT, although few trials reported consistently on these adverse events.
The USPSTF identified no studies on bupropion SR or varenicline pharmacotherapy during pregnancy. These drugs are both pregnancy category C, which means that animal reproduction studies have shown an adverse effect on the fetus but there are no adequate well-controlled studies in humans.
In the absence of clear evidence on the balance of benefits and harms of pharmacotherapy in pregnant women, clinicians are encouraged to consider the severity of smoking behavior in each patient and engage in shared decision making to determine the best individual treatment course.
ENDS. Approximately 69% of adults who smoke daily report interest in quitting, and roughly 43% attempted to quit in the previous year.1 To date, no ENDS manufacturer has applied for or received FDA approval to market its product for smoking cessation purposes. According to a small 2013 study, approximately two-thirds of physicians reported that they believed that electronic cigarettes (e-cigarettes) were a helpful aid for smoking cessation, and 35% recommended them to patients.12 A recent small survey of e-cigarette users found that 56% reported using them to quit or reduce cigarette use, and 26% reported using them to smoke in places where conventional cigarettes were banned.13 Because of the perception by the public and clinicians that ENDS may be used for quitting conventional smoking, the USPSTF reviewed the evidence in this area. No studies evaluated the use of ENDS for smoking cessation in pregnant women or adolescents. The USPSTF identified only 2 RCTs that evaluated the effect of e-cigarettes on smoking abstinence in adults and found mixed results. Neither study reported any serious adverse events related to ENDS use; however, potential concerns raised in other literature include the unknown safety and toxicity of their components and aerosols,14,15 and poisoning in children who mishandle nicotine cartridges.16 How the ingredients in ENDS may affect a fetus is also unknown. Overall, the USPSTF found the evidence on the use of ENDS as a smoking cessation tool in adults, including pregnant women, and adolescents to be insufficient.
ADDITIONAL APPROACHES TO PREVENTION
Given the public health significance of the consequences of tobacco use, numerous public health interventions aim to prevent tobacco use and promote smoking cessation. The Community Preventive Services Task Force offers several recommendations on interventions that can be used in community settings (available at http://www.thecommunityguide.org/tobacco/index.html). The Surgeon General's report, “The Health Consequences of Smoking—50 Years of Progress,” discusses initiatives to end the tobacco use epidemic in the United States.1 In addition, the USPSTF recommends that primary care clinicians provide interventions, including education or brief counseling, to prevent the initiation of tobacco use among school-aged children and adolescents (available at http://www.uspreventiveservicestaskforce.org).
This recommendation statement was first published in Ann Intern Med. 2015;163(8):622–634.
The “Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
