
Am Fam Physician. 2016;94(4):276-282
Related letter: Antipsychotic Use in Patients with Dementia with Lewy Bodies
Patient information: See related handout on behavior problems in patients with dementia.
Author disclosure: No relevant financial affiliations.
Behavioral and psychological symptoms of dementia pose management challenges for caregivers and clinicians. First-line nonpharmacologic treatments include eliminating physical and emotional stressors, modifying the patient's environment, and establishing daily routines. Family members and caregivers benefit from education about dementia symptoms and reminders that the behaviors are normal and unintentional. Cognitive and emotion-oriented interventions, sensory stimulation interventions, behavior management techniques, and other psychosocial interventions are modestly effective. In refractory cases, physicians may choose to prescribe off-label antipsychotics. Aripiprazole has the most consistent evidence of symptom improvement; however, this improvement is small. Olanzapine, quetiapine, and risperidone have inconsistent evidence of benefit. Physicians should use the smallest effective dose for the shortest possible duration to minimize adverse effects, most notably an increased mortality risk. Other adverse effects include anticholinergic and antidopaminergic effects, extrapyramidal symptoms, neuroleptic malignant syndrome, postural hypotension, metabolic syndrome, cardiac arrhythmia, and sedation. Patients should be monitored for these effects while receiving treatment; however, laboratory monitoring may be limited to patients receiving long-term therapy.
Behavioral and psychological symptoms of dementia pose management challenges for caregivers and clinicians. Although non-drug therapy is effective, Medicare drug claims in 14% of nursing home residents included atypical (second-generation) antipsychotic medication use for treating symptoms of dementia.1 This off-label use of antipsychotics occurs despite a U.S. Food and Drug Administration (FDA) boxed warning noting an increased risk of death when antipsychotics are used in patients with dementia-related psychosis.2 Because the estimated number of U.S. adults with dementia was 3.4 million in 2002 and is projected to double by 2025, primary care physicians should be familiar with nonpharmacologic management of dementia-related symptoms and with the effectiveness and risks of antipsychotic medications before initiating off-label use.3–5
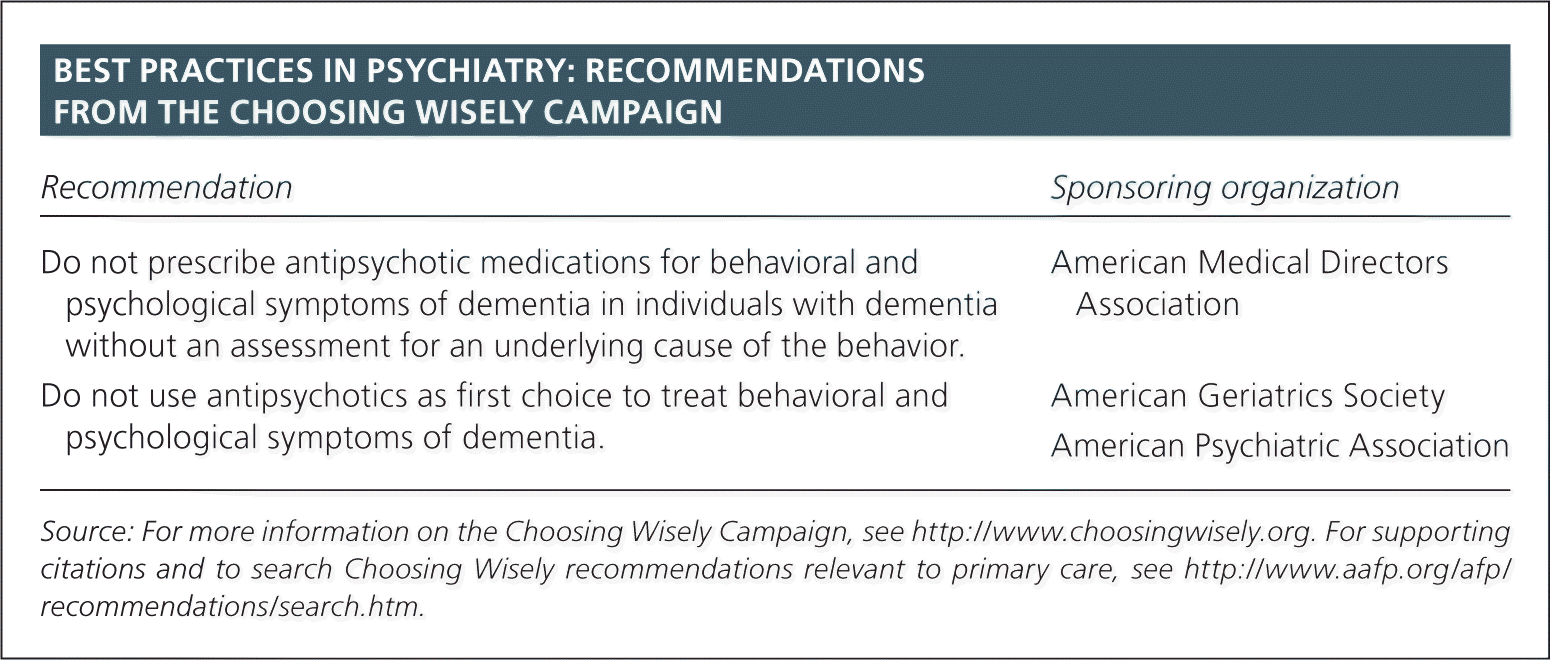
| Recommendation | Sponsoring organization |
|---|---|
| Do not prescribe antipsychotic medications for behavioral and psychological symptoms of dementia in individuals with dementia without an assessment for an underlying cause of the behavior. | American Medical Directors Association |
| Do not use antipsychotics as first choice to treat behavioral and psychological symptoms of dementia. | American Geriatrics Society American Psychiatric Association |
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| Nonpharmacologic interventions should be used as first-line treatment for behavioral and psychological symptoms of dementia. | C | 7, 13 |
| Before initiating antipsychotic therapy in older patients, physicians should have and document a discussion with patients and caregivers about the risks and benefits of these medications. | C | 2, 13, 14 |
| The use of atypical antipsychotics for behavioral and psychological symptoms of dementia is associated with increased mortality. | A | 23, 24 |
| Antipsychotic medications should be discontinued if there is no evidence of symptom improvement. | A | 13, 29, 30 |
Symptoms of Dementia
Approximately 15% to 75% of persons with dementia have delusions, delusional misidentifications, hallucinations (usually visual), wandering, agitation, aggression, and other psychotic behaviors.6 Collectively, these symptoms are referred to as behavioral and psychological symptoms of dementia. These symptoms contribute to caregiver fatigue and burnout, which often influence the decision to seek out-of-home care. These symptoms are often indicative of underlying emotional distress, pain, delirium, or physical discomforts, which can be treated supportively. Family and caregivers benefit from education about current and anticipated dementia symptoms and reminders that the behaviors are normal and unintentional.
Nonpharmacologic Management
The American Geriatrics Society and American Association for Geriatric Psychiatry consider nonpharmacologic interventions first-line treatment for behavioral and psychological symptoms of dementia.7 Physicians should assess for potential underlying causes of the concerning behaviors. Clinicians and caregivers should evaluate and treat physical discomforts such as thirst, hunger, pain, toileting difficulties, or nausea.8 Sleep deprivation, fatigue, depression, loneliness, boredom, overstimulation, and social stressors may serve as emotional triggers.7 Physical and emotional symptoms can be treated by establishing routines for toileting, eating, medication administration, sleep, and socialization. If routines do not correct the behaviors and safety concerns remain, physical barriers to wandering may be necessary.
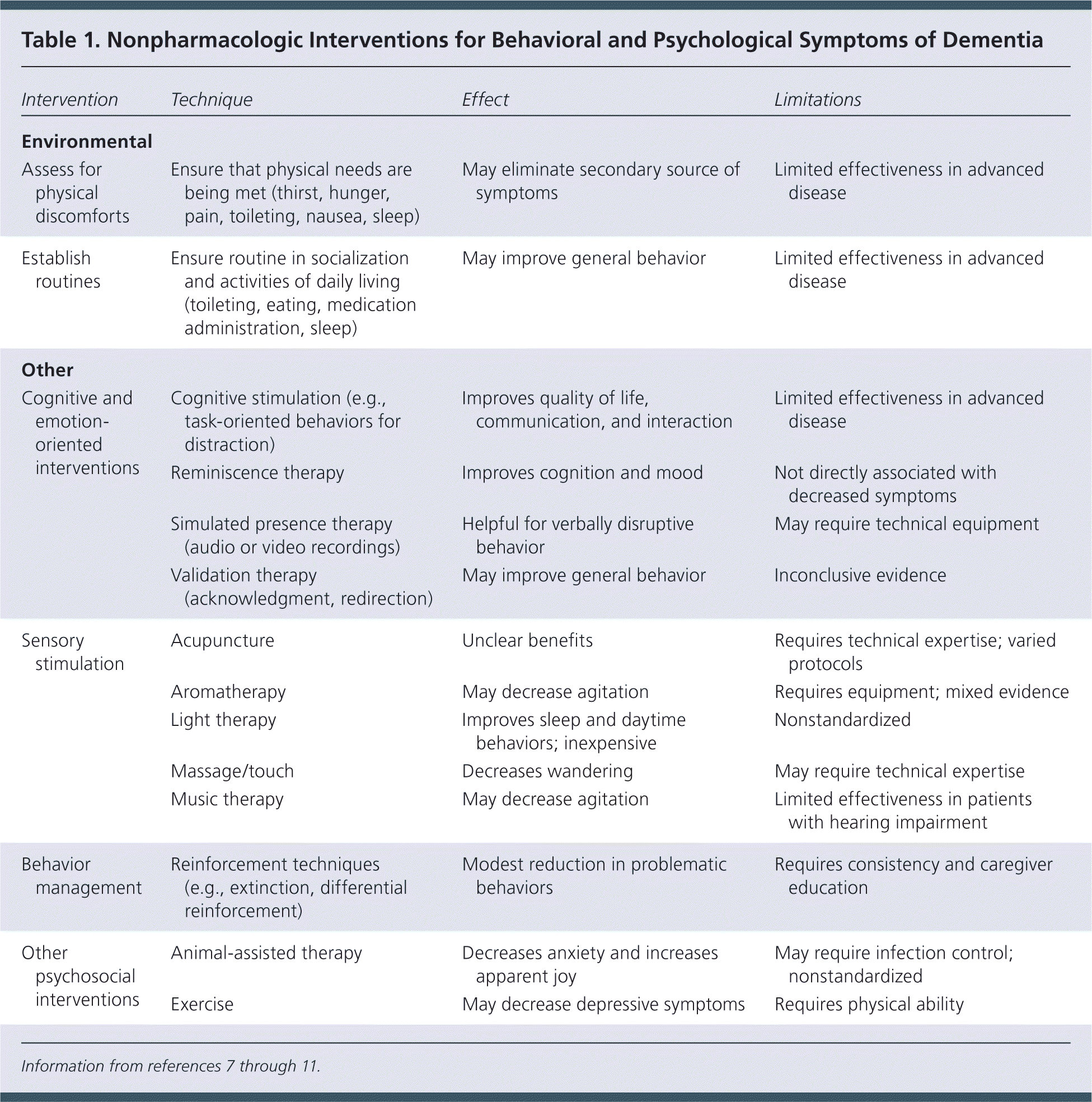
| Intervention | Technique | Effect | Limitations |
|---|---|---|---|
| Environmental | |||
| Assess for physical discomforts | Ensure that physical needs are being met (thirst, hunger, pain, toileting, nausea, sleep) | May eliminate secondary source of symptoms | Limited effectiveness in advanced disease |
| Establish routines | Ensure routine in socialization and activities of daily living (toileting, eating, medication administration, sleep) | May improve general behavior | Limited effectiveness in advanced disease |
| Other | |||
| Cognitive and emotion-oriented interventions | Cognitive stimulation (e.g., task-oriented behaviors for distraction) | Improves quality of life, communication, and interaction | Limited effectiveness in advanced disease |
| Reminiscence therapy | Improves cognition and mood | Not directly associated with decreased symptoms | |
| Simulated presence therapy (audio or video recordings) | Helpful for verbally disruptive behavior | May require technical equipment | |
| Validation therapy (acknowledgment, redirection) | May improve general behavior | Inconclusive evidence | |
| Sensory stimulation | Acupuncture | Unclear benefits | Requires technical expertise; varied protocols |
| Aromatherapy | May decrease agitation | Requires equipment; mixed evidence | |
| Light therapy | Improves sleep and daytime behaviors; inexpensive | Nonstandardized | |
| Massage/touch | Decreases wandering | May require technical expertise | |
| Music therapy | May decrease agitation | Limited effectiveness in patients with hearing impairment | |
| Behavior management | Reinforcement techniques (e.g., extinction, differential reinforcement) | Modest reduction in problematic behaviors | Requires consistency and caregiver education |
| Other psychosocial interventions | Animal-assisted therapy | Decreases anxiety and increases apparent joy | May require infection control; nonstandardized |
| Exercise | May decrease depressive symptoms | Requires physical ability | |
Cognitive and emotion-oriented interventions include cognitive stimulation, reminiscence therapy, simulated presence therapy, and validation therapy. Cognitive stimulation includes regularly scheduled mentally stimulating activities.10 During reminiscence therapy, the patient engages with another person or group in remembering past activities, events, or experiences.9 In simulated presence therapy, audio or audiovisual recordings are played to mimic the presence of a loved one. Validation therapy involves acknowledging the patient's current emotional state or voiced wishes and may include redirection from the unwanted behavior.
Sensory stimulation interventions include acupuncture, aromatherapy, light therapy, massage, and music therapy. Behavior management techniques include extinction and differential reinforcement. Extinction is withholding of positive reinforcement during inappropriate behavior. Differential reinforcement rewards quiet behavior or actions that are incompatible with the inappropriate behavior. Reinforcements can include social reinforcement, touch, food, and pleasurable activities.12 Other psychosocial interventions include animal-assisted therapy and exercise.10
Appropriate Antipsychotic Use and Safety
PRESCRIBING GUIDELINES
Antipsychotics are often used to treat refractory or severe behavioral and psychological symptoms of dementia, although the FDA has not approved this use because of low-quality evidence of benefit and good-quality evidence of harm.1,2 If first-line, nonpharmacologic therapies are ineffective, physicians should consider the risks and benefits of initiating off-label antipsychotic medications. Clinicians should initiate treatment only if the behaviors pose a risk of harm to the patient or others (e.g., hitting, verbal assaults, dangerous wandering) or if they are severely debilitating and other resources have been exhausted.13 Medication should be started at the lowest dose and titrated slowly.4 The risks, benefits, therapeutic goals, and adverse effects should be discussed with the patient and caregivers before therapy is initiated and again at every encounter.2,14 Written documentation of counseling sessions is essential for continuing assessment of treatment goals. Physicians may consider administering antipsychotics at bedtime to take advantage of their sedating effect.
EFFECTIVENESS
Olanzapine (Zyprexa; 5 mg per day), quetiapine (Seroquel; 50 mg per day), and risperidone (Risperdal; 0.25 to 1.5 mg per day) were inconsistently effective for the treatment of behavioral and psychological symptoms of dementia, with olanzapine and quetiapine having the least effect on symptom scores.15,16 Ineffective antipsychotics include ziprasidone (Geodon), paliperidone (Invega), clozapine (Clozaril), asenapine (Saphris), and iloperidone (Fanapt).15
ADVERSE EFFECTS
Antidopaminergic effects (e.g., movement disorders) are more common among first-generation antipsychotics but also occur with atypical antipsychotics (Table 218 and Table 319–22 ). Additional adverse effects include anticholinergic effects, extrapyramidal symptoms, neuroleptic malignant syndrome, hyperprolactinemia, postural hypotension, sedation, stroke, and prolonged QT interval. Long-term use of antipsychotics is associated with increased risk of metabolic syndrome, obesity, diabetes mellitus, hypertension, and dyslipidemia.18
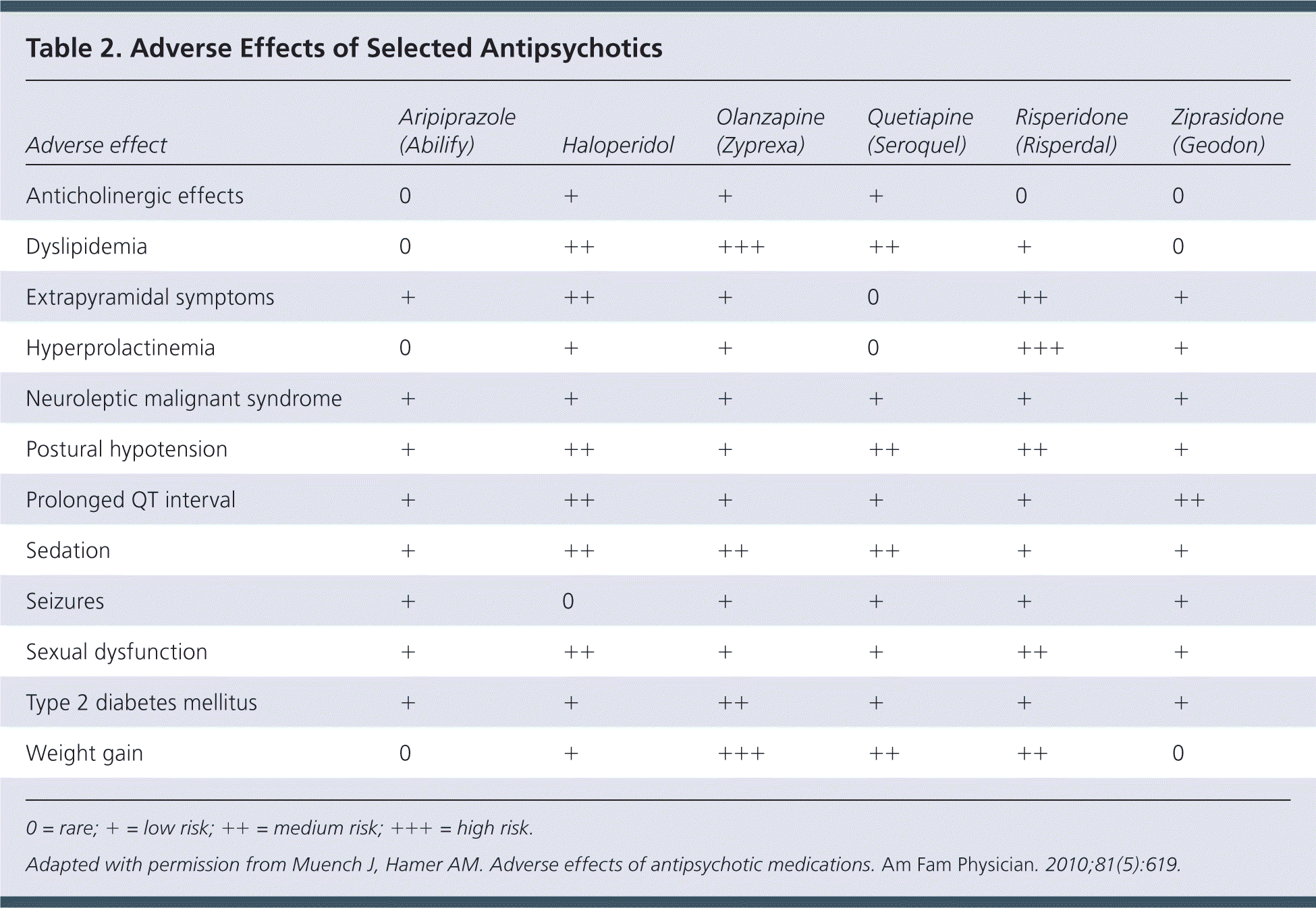
| Adverse effect | Aripiprazole (Abilify) | Haloperidol | Olanzapine (Zyprexa) | Quetiapine (Seroquel) | Risperidone (Risperdal) | Ziprasidone (Geodon) |
|---|---|---|---|---|---|---|
| Anticholinergic effects | 0 | + | + | + | 0 | 0 |
| Dyslipidemia | 0 | ++ | +++ | ++ | + | 0 |
| Extrapyramidal symptoms | + | ++ | + | 0 | ++ | + |
| Hyperprolactinemia | 0 | + | + | 0 | +++ | + |
| Neuroleptic malignant syndrome | + | + | + | + | + | + |
| Postural hypotension | + | ++ | + | ++ | ++ | + |
| Prolonged QT interval | + | ++ | + | + | + | ++ |
| Sedation | + | ++ | ++ | ++ | + | + |
| Seizures | + | 0 | + | + | + | + |
| Sexual dysfunction | + | ++ | + | + | ++ | + |
| Type 2 diabetes mellitus | + | + | ++ | + | + | + |
| Weight gain | 0 | + | +++ | ++ | ++ | 0 |
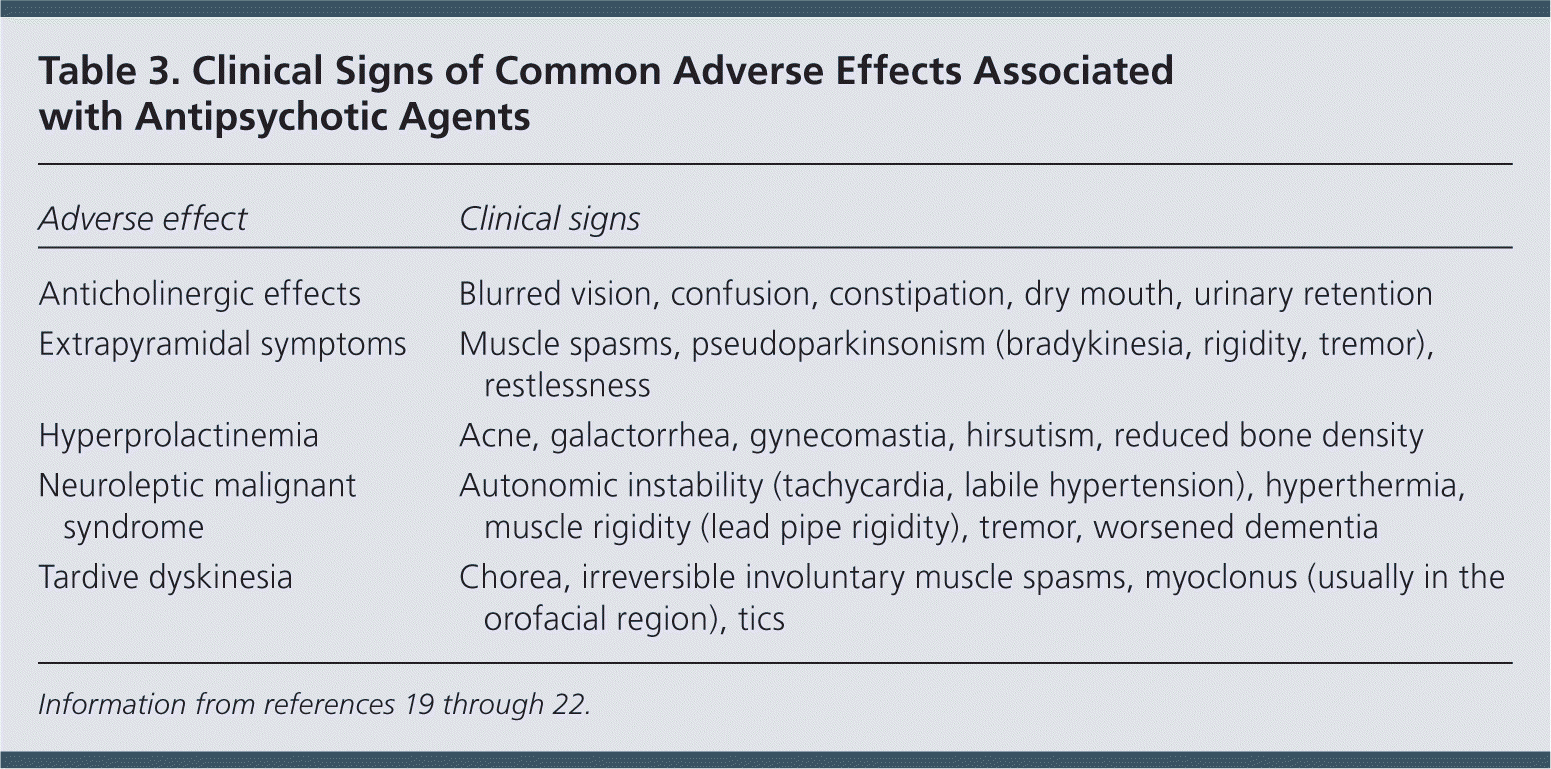
| Adverse effect | Clinical signs |
|---|---|
| Anticholinergic effects | Blurred vision, confusion, constipation, dry mouth, urinary retention |
| Extrapyramidal symptoms | Muscle spasms, pseudoparkinsonism (bradykinesia, rigidity, tremor), restlessness |
| Hyperprolactinemia | Acne, galactorrhea, gynecomastia, hirsutism, reduced bone density |
| Neuroleptic malignant syndrome | Autonomic instability (tachycardia, labile hypertension), hyperthermia, muscle rigidity (lead pipe rigidity), tremor, worsened dementia |
| Tardive dyskinesia | Chorea, irreversible involuntary muscle spasms, myoclonus (usually in the orofacial region), tics |
Although general adverse effects are significant, evidence of increased mortality is more concerning. In a 2015 retrospective case-control study of more than 90,000 Veteran's Administration beneficiaries with dementia, patients who received typical or atypical antipsychotics had a greater risk of death than those who did not receive these medications.23 The first-generation antipsychotic haloperidol had a number needed to treat to harm (NNH) of 26 (95% confidence interval [CI], 15 to 99). Of the second-generation antipsychotics studied, quetiapine increased mortality the least (NNH = 50; 95% CI, 30 to 150), followed by olanzapine (NNH = 40; 95% CI, 21 to 312) and risperidone (NNH = 27; 95% CI, 19 to 46). As a group, olanzapine, quetiapine, and risperidone had a 3.5% absolute increase in mortality (95% CI, 0.5% to 6.5%; NNH = 29) at higher vs. lower doses.24 Aripiprazole increases the risk of cardiac and cerebrovascular events (NNH = 58; 95% CI, 20 to 240), but its effect on mortality is not known.15
MONITORING AND DOSING ADJUSTMENT
The American Diabetes Association and American Psychiatric Association have guidelines for the long-term monitoring of patients receiving antipsychotics (Table 4).25,26 Physicians should consider the clinical context and anticipated duration of treatment when ordering these tests and monitoring effects. For example, patients with a life expectancy of less than 10 years may not benefit from monitoring for modifiable cardiovascular risk factors. However, regular assessment of dose-related adverse effects, such as extrapyramidal symptoms and sedation, may lead to dose reduction with acute benefits. Because older patients have decreased renal blood flow resulting in decreased renal clearance, assessment of renal function and subsequent dosing adjustments are key to reducing adverse effects in this population. Collaboration with a clinical pharmacist may help in reducing polypharmacy, monitoring for interactions, and making appropriate dose adjustments.27,28
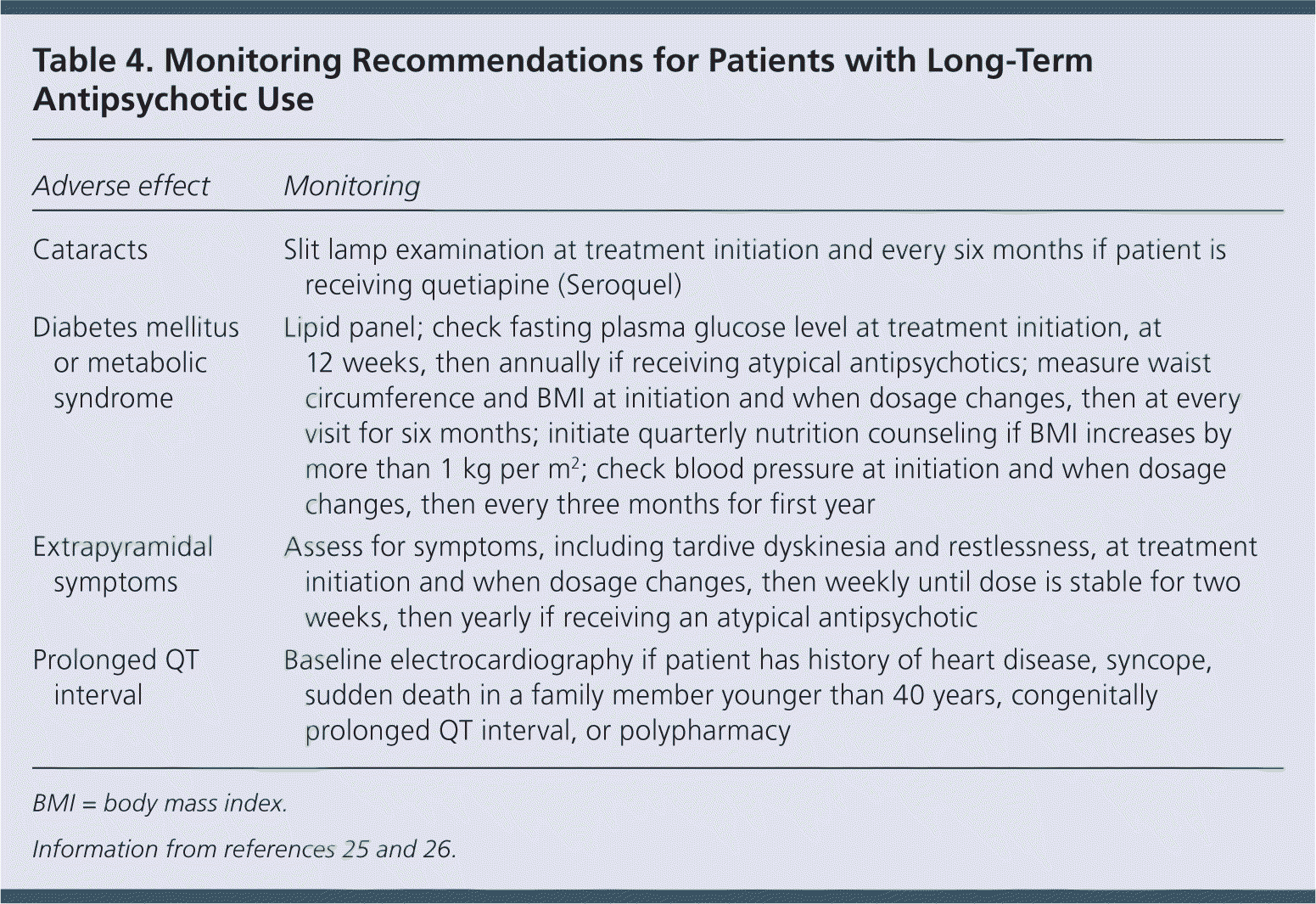
| Adverse effect | Monitoring |
|---|---|
| Cataracts | Slit lamp examination at treatment initiation and every six months if patient is receiving quetiapine (Seroquel) |
| Diabetes mellitus or metabolic syndrome | Lipid panel; check fasting plasma glucose level at treatment initiation, at 12 weeks, then annually if receiving atypical antipsychotics; measure waist circumference and BMI at initiation and when dosage changes, then at every visit for six months; initiate quarterly nutrition counseling if BMI increases by more than 1 kg per m2; check blood pressure at initiation and when dosage changes, then every three months for first year |
| Extrapyramidal symptoms | Assess for symptoms, including tardive dyskinesia and restlessness, at treatment initiation and when dosage changes, then weekly until dose is stable for two weeks, then yearly if receiving an atypical antipsychotic |
| Prolonged QT interval | Baseline electrocardiography if patient has history of heart disease, syncope, sudden death in a family member younger than 40 years, congenitally prolonged QT interval, or polypharmacy |
DURATION OF TREATMENT
A 2013 Cochrane review found that in eight of nine trials, there was no significant difference in behavioral and psychological symptoms of dementia after discontinuation of antipsychotics, indicating that the medications were ineffective in treating their target symptoms.29 Therefore, if symptoms do not improve after a four-week trial13 of antipsychotic medications, they should be discontinued.30 There is no evidence that discontinuation of antipsychotics has a negative effect on quality of life, ability to perform daily tasks, or intellectual processes of patients who have not benefited from treatment.
One trial demonstrated relapse of symptoms after medication discontinuation in patients who had successful symptom control, suggesting that some patients do benefit from antipsychotics beyond the short term.31 If patients' symptoms improve, clinicians should use the smallest dose for the shortest possible duration to reduce adverse effects.14
Data Sources: Searches were performed in PubMed, the Cochrane Database of Systematic Reviews, National Guideline Clearinghouse, Agency for Healthcare Research and Quality, and Essential Evidence. Key search words included antipsychotic, elderly, geriatric, dementia, delirium, geriatric psychiatry, agitation, and behavioral symptoms of dementia. Search dates: July 2015, September 2015, October 2015, January 2016, and April 2016.
The authors thank Diane Kunichika and Mark Ebell for their assistance with literature acquisition, and Muna Cocker for editorial oversight.
NOTE:
This article updates previous articles on this topic by Rayner, et al.,4 and Motsinger, et al.32
The views expressed in the manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. government.
