
Am Fam Physician. 2016;94(8):668-671
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• LAIV is not recommended this season because of its low effectiveness in recent years.
• Patients at risk of complications from influenza include persons 65 years and older, children younger than five years, and persons of any age who are immunosuppressed or have chronic medical conditions such as pulmonary disease.
• Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive any of the recommended age-appropriate influenza vaccines.
From the AFP Editors
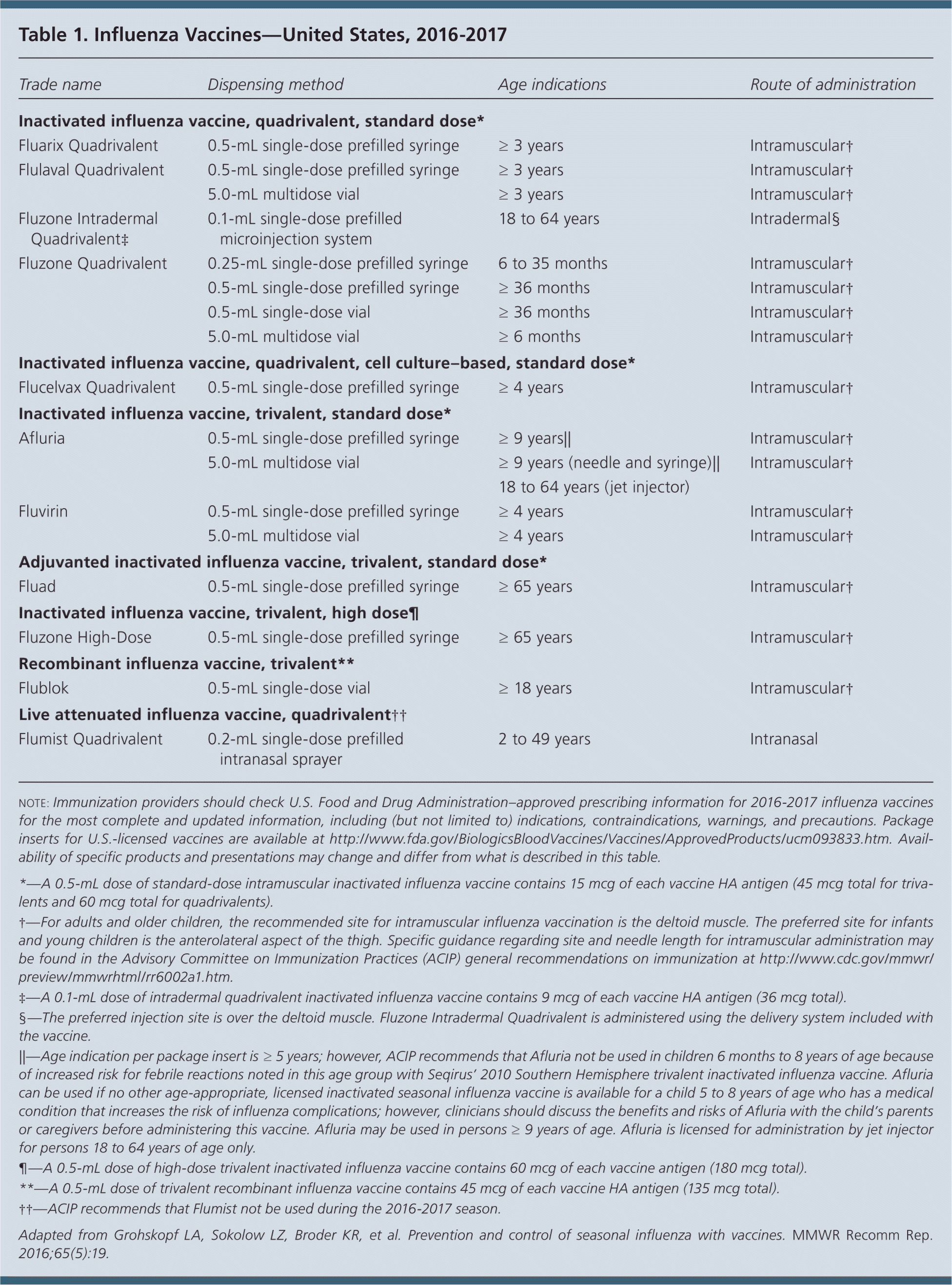
The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) has released its annual recommendations for routine influenza vaccination in the 2016–2017 season. Updates this year include the antigenic composition of seasonal influenza vaccines available in the United States; information on influenza vaccines expected to be available this season, including newly licensed products (Table 1); a recommendation against the use of live attenuated influenza vaccine (LAIV); and revised recommendations for vaccination of persons with egg allergy.
| Trade name | Dispensing method | Age indications | Route of administration |
|---|---|---|---|
| Inactivated influenza vaccine, quadrivalent, standard dose* | |||
| Fluarix Quadrivalent | 0.5-mL single-dose prefilled syringe | ≥ 3 years | Intramuscular† |
| Flulaval Quadrivalent | 0.5-mL single-dose prefilled syringe | ≥ 3 years | Intramuscular† |
| 5.0-mL multidose vial | ≥ 3 years | Intramuscular† | |
| Fluzone Intradermal Quadrivalent‡ | 0.1-mL single-dose prefilled microinjection system | 18 to 64 years | Intradermal§ |
| Fluzone Quadrivalent | 0.25-mL single-dose prefilled syringe | 6 to 35 months | Intramuscular† |
| 0.5-mL single-dose prefilled syringe | ≥ 36 months | Intramuscular† | |
| 0.5-mL single-dose vial | ≥ 36 months | Intramuscular† | |
| 5.0-mL multidose vial | ≥ 6 months | Intramuscular† | |
| Inactivated influenza vaccine, quadrivalent, cell culture–based, standard dose* | |||
| Flucelvax Quadrivalent | 0.5-mL single-dose prefilled syringe | ≥ 4 years | Intramuscular† |
| Inactivated influenza vaccine, trivalent, standard dose* | |||
| Afluria | 0.5-mL single-dose prefilled syringe | ≥ 9 years|| | Intramuscular† |
| 5.0-mL multidose vial | ≥ 9 years (needle and syringe)|| | Intramuscular† | |
| 18 to 64 years (jet injector) | |||
| Fluvirin | 0.5-mL single-dose prefilled syringe | ≥ 4 years | Intramuscular† |
| 5.0-mL multidose vial | ≥ 4 years | Intramuscular† | |
| Adjuvanted inactivated influenza vaccine, trivalent, standard dose* | |||
| Fluad | 0.5-mL single-dose prefilled syringe | ≥ 65 years | Intramuscular† |
| Inactivated influenza vaccine, trivalent, high dose¶ | |||
| Fluzone High-Dose | 0.5-mL single-dose prefilled syringe | ≥ 65 years | Intramuscular† |
| Recombinant influenza vaccine, trivalent** | |||
| Flublok | 0.5-mL single-dose vial | ≥ 18 years | Intramuscular† |
| Live attenuated influenza vaccine, quadrivalent†† | |||
| Flumist Quadrivalent | 0.2-mL single-dose prefilled intranasal sprayer | 2 to 49 years | Intranasal |
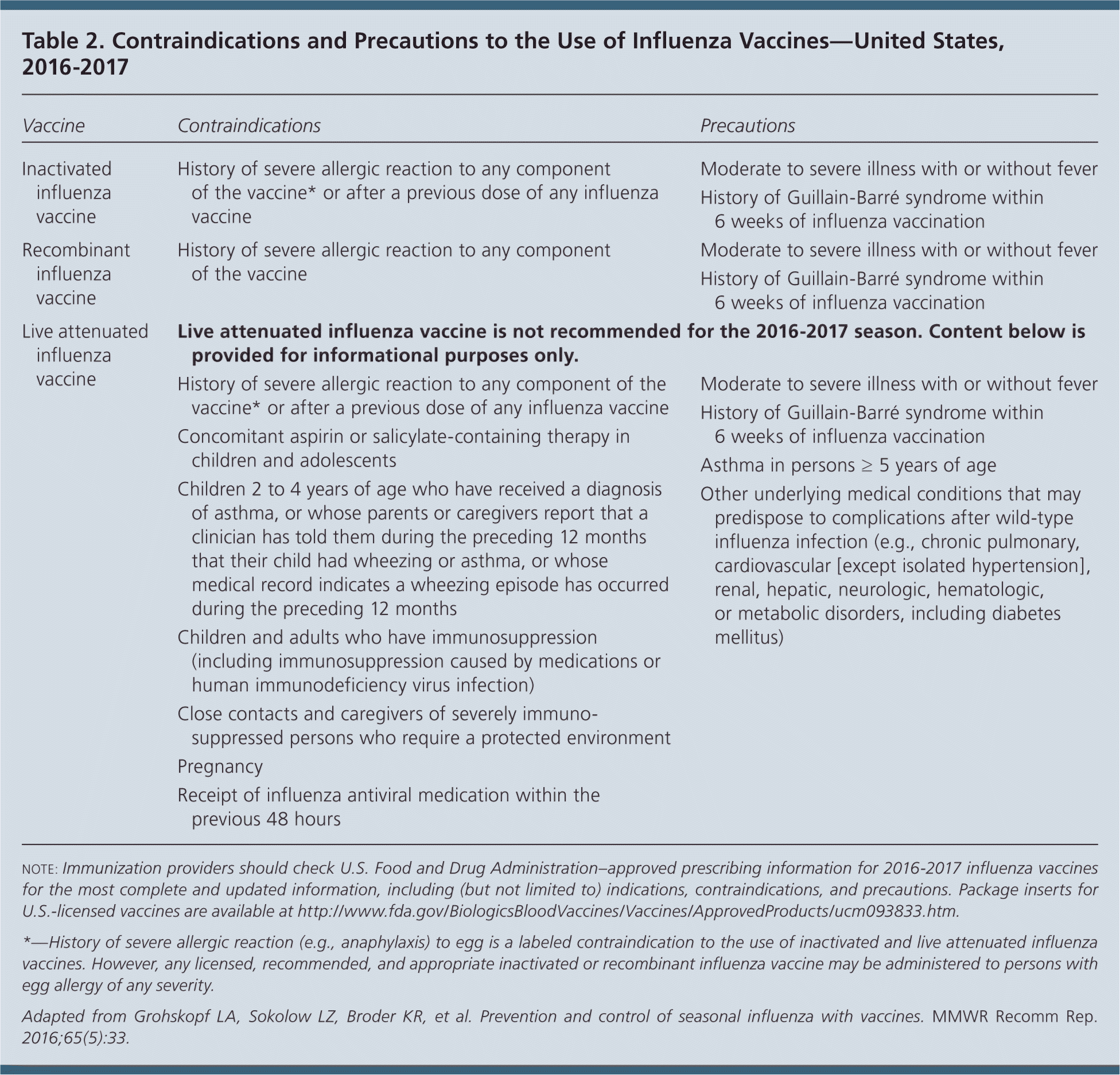
Routine annual influenza vaccination is recommended for all persons six months and older who do not have contraindications. No single vaccine is preferred over others in persons for whom more than one product is appropriate (Table 2). The composition of influenza vaccines typically changes from season to season, with one or more vaccine strains replaced annually to provide protection against viruses that are anticipated to circulate during the upcoming season. Clinical trials have shown that protection against viruses that are antigenically similar to those in the vaccine extends at least for six to eight months, particularly in nonelderly populations. Influenza strains contained in this year's trivalent vaccines include an A/California/7/2009 (H1N1)-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage). Quadrivalent vaccines will include an additional strain, a B/Phuket/3073/2013–like virus (Yamagata lineage). These vaccines include a change in the H3N2 virus and the lineage of the influenza B viruses.
ACIP recommends that quadrivalent LAIV not be used this season because of its low effectiveness in recent years. However, it remains a licensed vaccine that, if available, some clinicians may elect to use; for this reason, previous recommendations for its use are provided in Table 2 for informational purposes.
| Vaccine | Contraindications | Precautions |
|---|---|---|
| Inactivated influenza vaccine | History of severe allergic reaction to any component of the vaccine* or after a previous dose of any influenza vaccine | Moderate to severe illness with or without fever |
| History of Guillain-Barré syndrome within 6 weeks of influenza vaccination | ||
| Recombinant influenza vaccine | History of severe allergic reaction to any component of the vaccine | Moderate to severe illness with or without fever |
| History of Guillain-Barré syndrome within 6 weeks of influenza vaccination | ||
| Live attenuated influenza vaccine | Live attenuated influenza vaccine is not recommended for the 2016–2017 season. Content below is provided for informational purposes only. | |
| History of severe allergic reaction to any component of the vaccine* or after a previous dose of any influenza vaccine | Moderate to severe illness with or without fever | |
| Concomitant aspirin or salicylate-containing therapy in children and adolescents | History of Guillain-Barré syndrome within 6 weeks of influenza vaccination | |
| Asthma in persons ≥ 5 years of age | ||
| Children 2 to 4 years of age who have received a diagnosis of asthma, or whose parents or caregivers report that a clinician has told them during the preceding 12 months that their child had wheezing or asthma, or whose medical record indicates a wheezing episode has occurred during the preceding 12 months | Other underlying medical conditions that may predispose to complications after wild-type influenza infection (e.g., chronic pulmonary, cardiovascular [except isolated hypertension], renal, hepatic, neurologic, hematologic, or metabolic disorders, including diabetes mellitus) | |
| Children and adults who have immunosuppression (including immunosuppression caused by medications or human immunodeficiency virus infection) | ||
| Close contacts and caregivers of severely immunosuppressed persons who require a protected environment | ||
| Pregnancy | ||
| Receipt of influenza antiviral medication within the previous 48 hours | ||
Although the precise timing of the onset, peak, and end of influenza activity varies from one season to the next, annual epidemics of seasonal influenza typically occur in the United States between October and April. Ideally, vaccination should occur before the onset of influenza activity in the community. Clinicians should offer vaccination by the end of October, if possible. Children six months to eight years of age who have not received influenza vaccination before require two doses for the first season. They should receive their first dose as soon as vaccine becomes available, followed by a second vaccination no earlier than four weeks later. In recent years, some clinicians have received their initial shipments of vaccine as early as July. This has raised questions about the ideal timing of vaccination because early availability and administration of vaccine—particularly in older adults—may contribute to reduced protection against influenza later in the season. Conversely, delaying vaccination may confer greater immunity later in the season, but may also result in missed opportunities to vaccinate.
Persons of all ages are susceptible to seasonal influenza, but complications, hospitalizations, and deaths are typically greatest among persons 65 years and older, children younger than five years (especially those younger than two years), and persons of any age who are immunosuppressed or have certain medical conditions, such as chronic cardiovascular, pulmonary, or renal disease. Vaccination is particularly important in these populations. When vaccine supply is limited, vaccination efforts should target the following persons:
Children six to 59 months of age
Children and adolescents six months to 18 years of age who are receiving long-term aspirin therapy and may be at risk of Reye syndrome after influenza virus infection
Pregnant women
Adults 50 years and older
Residents of nursing homes and long-term care facilities
American Indians/Alaska Natives
Persons with a body mass index of 40 kg per m2 or greater
Adults and children with chronic pulmonary, cardiovascular, renal, hepatic, neurologic, hematologic, or metabolic disorders, such as asthma and diabetes mellitus
Persons with immunosuppression.
Severe allergic reactions, including anaphylaxis, can occur in response to components of all vaccines; however, these reactions are rare. Not all reactions are related to egg proteins, but the possibility of reactions to influenza vaccines in persons with egg allergy may be of concern. With the exceptions of recombinant trivalent influenza vaccine (Flublok) and cell culture–based quadrivalent inactivated influenza vaccine (Flucelvax Quadrivalent), currently available influenza vaccines are prepared by propagation of virus in embryonated eggs. However, evidence indicates that severe allergic reactions are unlikely. Therefore, ACIP recommends that persons with a history of egg allergy who have experienced only hives after exposure to egg should receive any of the recommended influenza vaccines appropriate for the recipient's age and health status. Persons who have had reactions to egg involving symptoms other than hives (e.g., angioedema, respiratory distress, lightheadedness, recurrent emesis) or who required epinephrine or another emergency medical intervention may receive the cell culture–based or recombinant influenza vaccines appropriate for the recipient's age and health status. The selected vaccine should be administered in an inpatient or outpatient setting and should be supervised by a clinician who is able to recognize and manage severe allergic conditions. A previous severe allergic reaction to influenza vaccine is a contraindication to future receipt of the vaccine.
ACIP has removed its previous recommendation that persons allergic to eggs should be observed for 30 minutes after receipt of influenza vaccine. However, clinicians should consider observing all patients—whether or not they are allergic to egg—for 15 minutes postvaccination to decrease the risk of injury if they experience syncope.
Guideline source: Advisory Committee on Immunization Practices
Evidence rating system used? No
Literature search described? No
Guideline developed by participants without relevant financial ties to industry? No
Published source: MMWR Recomm Rep. August 26, 2016;65(5):1–54