Am Fam Physician. 2016;94(9):708-716
Related editorial: Primary Care for Patients with HIV Infection: It's Not Who Should Provide It, It's How to Provide It.
A more recent article on initial management of HIV in adults is available.
Author disclosure: No relevant financial affiliations.
Human immunodeficiency virus (HIV) infection has become a treatable chronic disease with near-normal life expectancy when patients receive antiretroviral therapy (ART). Family physicians and other primary care clinicians commonly provide long-term comprehensive care for persons with HIV infection. This article describes the scope of initial care, including obtaining a thorough history; physical examination for HIV-associated manifestations; attention to HIV-specific immunization schedules; routine and HIV-specific laboratory evaluation; and ensuring standard health care maintenance to prevent HIV- and non–HIV-related morbidity and mortality. Clinicians should encourage combination ART as early as possible, although careful assessment of patient readiness and ability to sustain lifelong treatment must be weighed. After ART initiation, monitoring viral load and CD4 lymphocyte response is essential to ensure viral suppression and evaluate immune system restoration. Opportunistic infections are now less common than in the past because ART usually prevents or markedly delays progression to advanced HIV disease. The most important reasons for consultation or comanagement with an HIV expert include management of antiretroviral drug resistance or drug toxicities, as well as special circumstances such as viral hepatitis coinfection or pregnancy.
Remarkable advancements in anti-retroviral therapy (ART) over the past 35 years have resulted in human immunodeficiency virus (HIV) infection becoming a manageable chronic disease.1 Patients receiving early suppressive ART now have near-normal life expectancies,2,3 reduced HIV-induced inflammation and chronic immune activation,4,5 decreased progression to advanced HIV disease,6 and markedly reduced risk of transmission.7
WHAT IS NEW ON THIS TOPIC: MANAGEMENT OF PATIENTS WITH HIV INFECTION
Management of patients with HIV infection, often in consultation with experts for selection or change of the ART regimen, is in the domain of primary care clinicians.
Current recommendations are to start ART as early as possible. Once-daily pill regimens are now available.
Effective ART markedly reduces the risk of HIV transmission.
ART = antiretroviral therapy; HIV = human immunodeficiency virus.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Combination ART should be initiated early to delay progression of HIV infection. | A | 4, 5 |
| Combination ART can prevent HIV transmission. | A | 7 |
| Patients with HIV infection who have substantial immunosuppression should receive prophylaxis to prevent or delay opportunistic infections. | C | 14 |
| After ART-induced immunologic improvement (as measured by increased CD4 lymphocyte counts), it is safe to discontinue prophylaxis for opportunistic infections. | C | 14 |
| Non–HIV-specific primary care should be provided for all persons with HIV infection. | C | 11, 12, 25 |
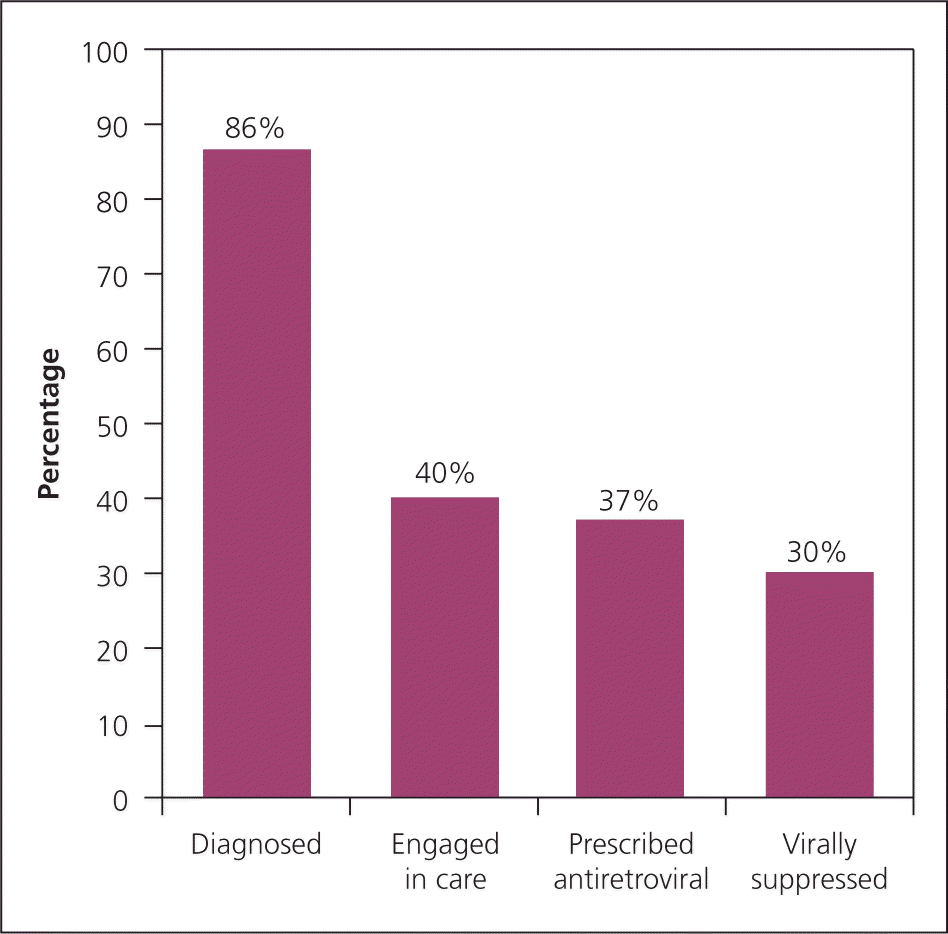
The HIV continuum of care illustrates falloff points in care, but also shows opportunities for intervention by primary care clinicians (Figure 1).8,9 There are 1.2 million persons infected with HIV in the United States, and as many as 70% are not receiving optimal care.9,10 Patients who are HIV-positive are often seen in the primary care setting because of a new HIV diagnosis or changes in health care coverage. This article, targeted toward primary care clinicians, describes the scope of initial care, including obtaining a thorough history and physical examination for HIV-associated manifestations; attention to HIV-specific immunization schedules; routine and HIV-specific laboratory evaluation; and ensuring standard health care maintenance to prevent HIV- and non–HIV-related morbidity and mortality.
Initial History and Physical Examination
The goal of the initial history and physical examination is to assess for clinical manifestations of HIV infection and related conditions. The initial history should elicit details about sexually transmitted infections, hepatitis, substance use, and sexual practices. It should include any history of previous HIV treatment and information about the patient's family and social networks. Except in cases of acute or advanced HIV infection, the initial physical examination generally shows no HIV-related manifestations. The presence of thrush, oral or anogenital ulcers or warts, lymphadenopathy, rashes, or skin lesions should prompt further evaluation,11 especially when persistent or substantial immunosuppression is present (as indicated by a CD4 lymphocyte count less than 200 cells per mm3 [0.20 × 109 per L]).
Initial Laboratory Testing
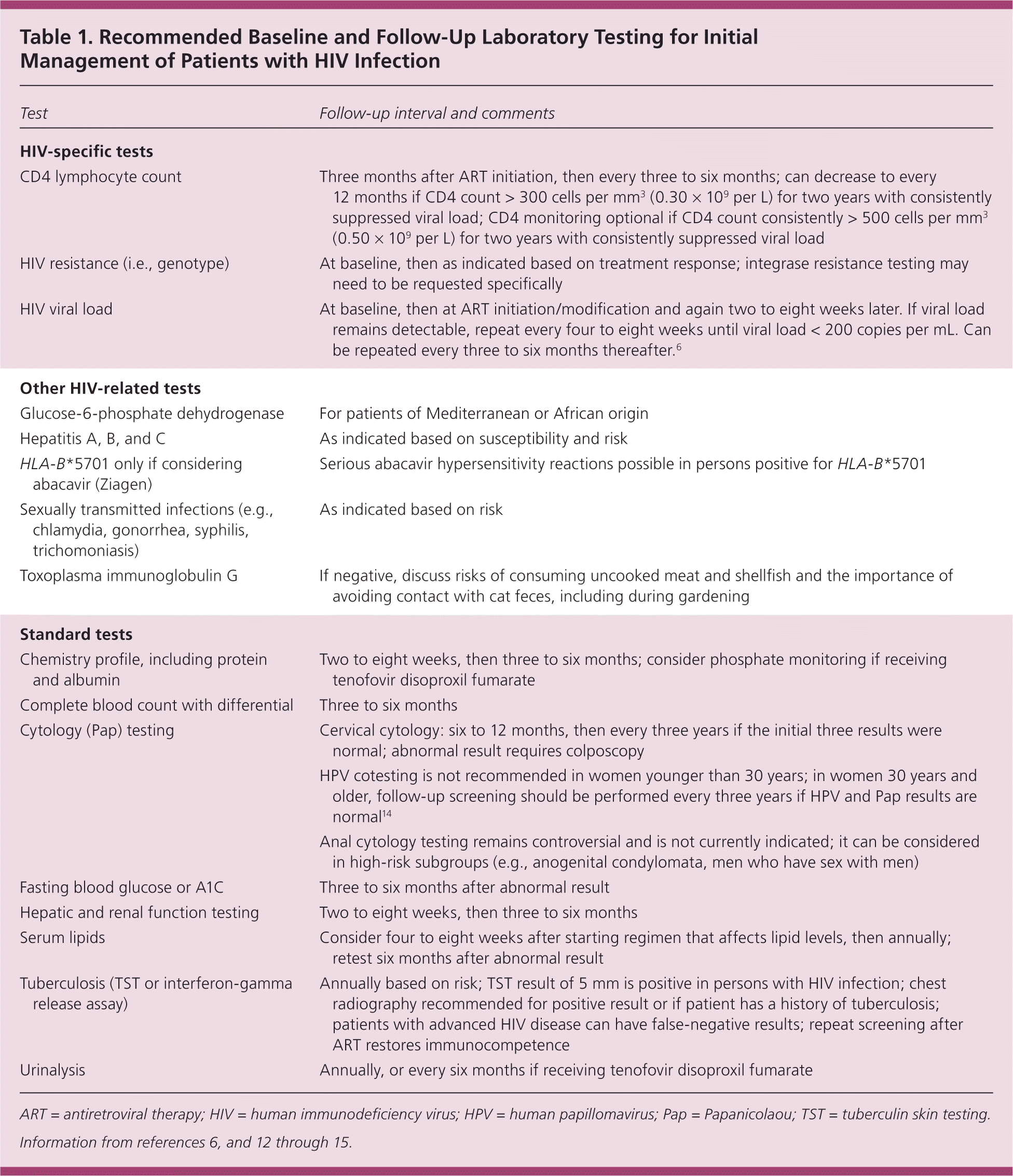
Initial laboratory testing (Table 1) includes standard primary care age- and sex-specific testing.6,12–15 HIV-specific baseline laboratory testing includes CD4 count, HIV viral load, and HIV drug resistance testing. The CD4 count is the most accurate measurement of the degree of immune system impairment, roughly correlates with duration and activity of HIV infection, and is the best predictor of prognosis. Untreated HIV infection generally has a 10-year progression until opportunistic infections and malignancies appear, usually when the CD4 count decreases to less than 200 cells per mm3.16 Normal CD4 counts are 1,000 ± 200 cells per mm3, but there is person-to-person and diurnal variability.6 CD4 counts should be interpreted cautiously during acute illnesses such as influenza or pyelonephritis, which may transiently decrease CD4 counts.
| Test | Follow-up interval and comments |
|---|---|
| HIV-specific tests | |
| CD4 lymphocyte count | Three months after ART initiation, then every three to six months; can decrease to every 12 months if CD4 count > 300 cells per mm3 (0.30 × 109 per L) for two years with consistently suppressed viral load; CD4 monitoring optional if CD4 count consistently > 500 cells per mm3 (0.50 × 109 per L) for two years with consistently suppressed viral load |
| HIV resistance (i.e., genotype) | At baseline, then as indicated based on treatment response; integrase resistance testing may need to be requested specifically |
| HIV viral load | At baseline, then at ART initiation/modification and again two to eight weeks later. If viral load remains detectable, repeat every four to eight weeks until viral load < 200 copies per mL. Can be repeated every three to six months thereafter.6 |
| Other HIV-related tests | |
| Glucose-6-phosphate dehydrogenase | For patients of Mediterranean or African origin |
| Hepatitis A, B, and C | As indicated based on susceptibility and risk |
| HLA-B*5701 only if considering abacavir (Ziagen) | Serious abacavir hypersensitivity reactions possible in persons positive for HLA-B*5701 |
| Sexually transmitted infections (e.g., chlamydia, gonorrhea, syphilis, trichomoniasis) | As indicated based on risk |
| Toxoplasma immunoglobulin G | If negative, discuss risks of consuming uncooked meat and shellfish and the importance of avoiding contact with cat feces, including during gardening |
| Standard tests | |
| Chemistry profile, including protein and albumin | Two to eight weeks, then three to six months; consider phosphate monitoring if receiving tenofovir disoproxil fumarate |
| Complete blood count with differential | Three to six months |
| Cytology (Pap) testing | Cervical cytology: six to 12 months, then every three years if the initial three results were normal; abnormal result requires colposcopy |
| HPV cotesting is not recommended in women younger than 30 years; in women 30 years and older, follow-up screening should be performed every three years if HPV and Pap results are normal14 | |
| Anal cytology testing remains controversial and is not currently indicated; it can be considered in high-risk subgroups (e.g., anogenital condylomata, men who have sex with men) | |
| Fasting blood glucose or A1C | Three to six months after abnormal result |
| Hepatic and renal function testing | Two to eight weeks, then three to six months |
| Serum lipids | Consider four to eight weeks after starting regimen that affects lipid levels, then annually; retest six months after abnormal result |
| Tuberculosis (TST or interferon-gamma release assay) | Annually based on risk; TST result of 5 mm is positive in persons with HIV infection; chest radiography recommended for positive result or if patient has a history of tuberculosis; patients with advanced HIV disease can have false-negative results; repeat screening after ART restores immunocompetence |
| Urinalysis | Annually, or every six months if receiving tenofovir disoproxil fumarate |
The viral load is a key predictor and indicator of treatment response. Acute illness or recent vaccination can modestly increase the viral load. HIV drug resistance testing (preferably a genotype) should be performed at entry into care. Baseline resistance testing provides information on whether antiretroviral drug resistance–associated mutations are present, guiding initial regimen selection. Resistance tests can be performed if the viral load is above the laboratory threshold (usually 500 copies per mL).
Screening
Routine age- and sex-specific screening should be performed for all patients with HIV infection. Screening for chronic diseases, such as hypertension, diabetes mellitus, dyslipidemia, colorectal cancer, and depression, is indicated.12 HIV-specific laboratory screening recommendations are listed in Table 1.6,12–15 Tuberculosis screening for patients without a history of tuberculosis or positive tuberculosis screening is particularly important because tuberculosis can occur at any CD4 level.
Immunizations
Immunizations should be kept up to date (Table 2).13 However, patients with low CD4 counts may not have optimal responses. Nonemergent vaccination can be deferred until ART-induced immune system restoration. Patients with HIV infection who have CD4 counts less than 200 cells per mm3 should not receive live vaccines (e.g., oral polio, live attenuated influenza, or varicella vaccine).13

| Vaccine | Dosing |
|---|---|
| Hepatitis A | Indicated when identified risk present* |
| Hepatitis B | Standard schedule† |
| Herpes zoster | Contraindicated if CD4 lymphocyte count < 200 cells per mm3 (0.20 × 109 per L), otherwise standard schedule |
| Human papillomavirus | Standard schedule‡ |
| Influenza (inactivated only) | Standard schedule |
| Measles, mumps, rubella | Contraindicated if CD4 count < 200 cells per mm3, otherwise standard schedule |
| Meningococcal | Indicated when identified risk present§ |
| Pneumococcal | Standard schedule unless previously immunized|| |
| Tetanus, diphtheria, pertussis | Standard schedule |
| Varicella | Contraindicated if CD4 count < 200 cells per mm3, otherwise standard schedule |
Antiretroviral Therapy
Current recommendations are to start ART as early as possible,6 particularly for patients with low CD4 counts. Initiating ART is a critical juncture in patients' lives, requiring thoughtful and realistic decision making. The clinician and patient should assess the likelihood that the patient can maintain daily lifetime therapy. Adherence is a strong predictor of ART effectiveness, and suboptimal medication adherence can produce permanent resistance to an entire class of antiretroviral medications.17 Clinicians should proactively identify and address patient concerns, including fears that taking antiretroviral drugs may disclose their diagnosis to others. Many patients are eager to start treatment immediately and have no factors limiting that decision. Patient preferences regarding pill burden, dosing considerations, and adverse effects should be discussed. It is essential to assess whether medication costs will be fully covered and whether the patient can obtain medications without interruption. Linking patients with social services, case management, HIV education, and counseling is critical. When there is reasonable concern that medication adherence will be a challenge, the clinician and patient should identify and address barriers to treatment (e.g., unstable housing, behavioral health issues, overall patient readiness) and consider deferring treatment until there is a greater likelihood of adherence.
Choosing an individualized initial ART regimen is based on ART resistance patterns, adverse effect pro-files, allergies, drug interaction potential, hepatic and renal function, presence of viral hepatitis coinfection or other comorbidities, and patient preferences about dosing frequency and pill burden. CD4 count, viral load, and recent (within the past three months) HIV resistance testing results should be available before initiating ART. Laboratory turnaround time for resistance testing is usually two to three weeks. If the decision is made to initiate ART before resistance testing results are known, expert consultation is recommended. If necessary, the regimen can be changed after results are available.
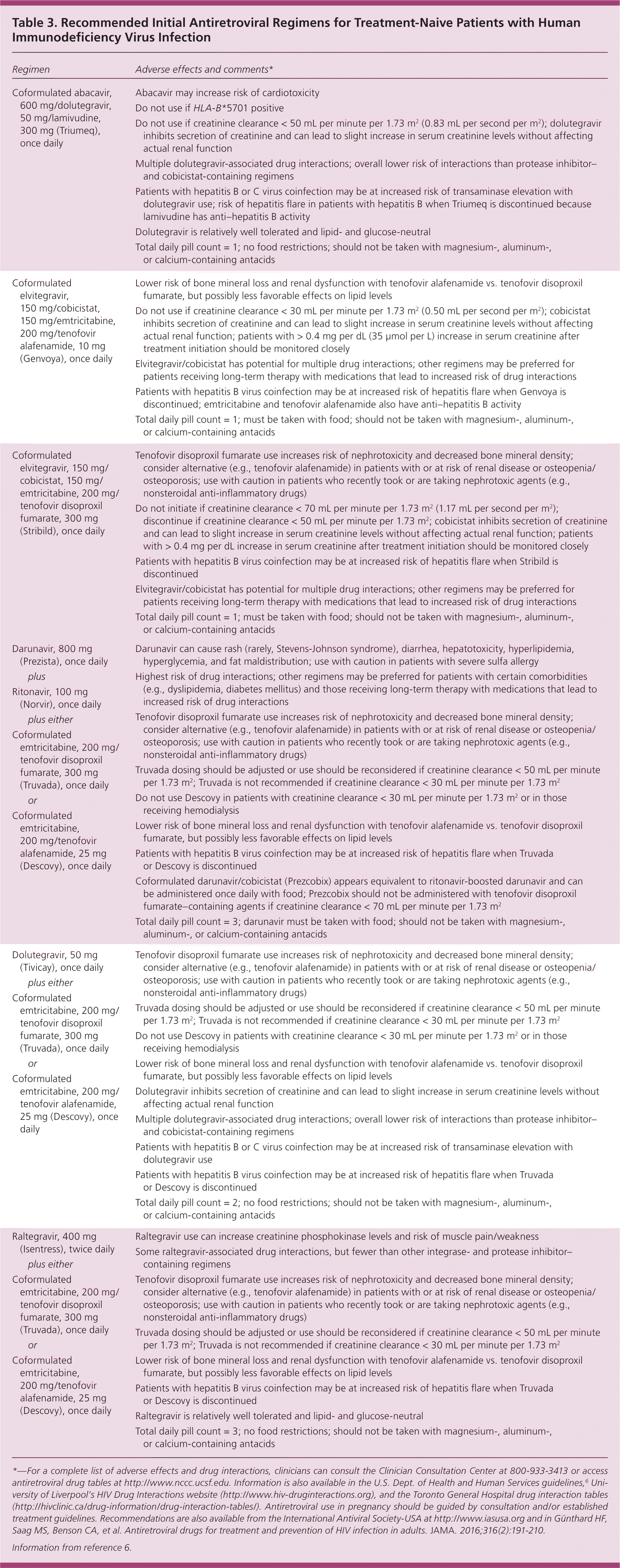
Recommended regimens for initial treatment in ART-naive patients include integrase inhibitor–based (dolutegravir, elvitegravir, and raltegravir) and protease inhibitor–based (darunavir) regimens (Table 3).6 Integrase-based combinations generally are better tolerated and have fewer drug interactions. All recommended regimens contain tenofovir, either as tenofovir disoproxil fumarate or its prodrug tenofovir alafenamide. Tenofovir alafenamide is associated with less bone and renal toxicity and can be used in lieu of tenofovir disoproxil fumarate when these effects are a concern. Once-daily and single-pill options are available.18 Treatment guidelines are updated at least annually.
| Regimen | Adverse effects and comments* | |
|---|---|---|
| Coformulated abacavir, 600 mg/dolutegravir, 50 mg/lamivudine, 300 mg (Triumeq), once daily | Abacavir may increase risk of cardiotoxicity | |
| Do not use if HLA-B*5701 positive | ||
| Do not use if creatinine clearance < 50 mL per minute per 1.73 m2 (0.83 mL per second per m2); dolutegravir inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting actual renal function | ||
| Multiple dolutegravir-associated drug interactions; overall lower risk of interactions than protease inhibitor– and cobicistat-containing regimens | ||
| Patients with hepatitis B or C virus coinfection may be at increased risk of transaminase elevation with dolutegravir use; risk of hepatitis flare in patients with hepatitis B when Triumeq is discontinued because lamivudine has anti–hepatitis B activity | ||
| Dolutegravir is relatively well tolerated and lipid- and glucose-neutral | ||
| Total daily pill count = 1; no food restrictions; should not be taken with magnesium-, aluminum-, or calcium-containing antacids | ||
| Coformulated elvitegravir, 150 mg/cobicistat, 150 mg/emtricitabine, 200 mg/tenofovir alafenamide, 10 mg (Genvoya), once daily | Lower risk of bone mineral loss and renal dysfunction with tenofovir alafenamide vs. tenofovir disoproxil fumarate, but possibly less favorable effects on lipid levels | |
| Do not use if creatinine clearance < 30 mL per minute per 1.73 m2 (0.50 mL per second per m2); cobicistat inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting actual renal function; patients with > 0.4 mg per dL (35 μmol per L) increase in serum creatinine after treatment initiation should be monitored closely | ||
| Elvitegravir/cobicistat has potential for multiple drug interactions; other regimens may be preferred for patients receiving long-term therapy with medications that lead to increased risk of drug interactions | ||
| Patients with hepatitis B virus coinfection may be at increased risk of hepatitis flare when Genvoya is discontinued; emtricitabine and tenofovir alafenamide also have anti–hepatitis B activity | ||
| Total daily pill count = 1; must be taken with food; should not be taken with magnesium-, aluminum-, or calcium-containing antacids | ||
| Coformulated elvitegravir, 150 mg/cobicistat, 150 mg/emtricitabine, 200 mg/tenofovir disoproxil fumarate, 300 mg (Stribild), once daily | Tenofovir disoproxil fumarate use increases risk of nephrotoxicity and decreased bone mineral density; consider alternative (e.g., tenofovir alafenamide) in patients with or at risk of renal disease or osteopenia/osteoporosis; use with caution in patients who recently took or are taking nephrotoxic agents (e.g., nonsteroidal anti-inflammatory drugs) | |
| Do not initiate if creatinine clearance < 70 mL per minute per 1.73 m2 (1.17 mL per second per m2); discontinue if creatinine clearance < 50 mL per minute per 1.73 m2; cobicistat inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting actual renal function; patients with > 0.4 mg per dL increase in serum creatinine after treatment initiation should be monitored closely | ||
| Patients with hepatitis B virus coinfection may be at increased risk of hepatitis flare when Stribild is discontinued | ||
| Elvitegravir/cobicistat has potential for multiple drug interactions; other regimens may be preferred for patients receiving long-term therapy with medications that lead to increased risk of drug interactions | ||
| Total daily pill count = 1; must be taken with food; should not be taken with magnesium-, aluminum-, or calcium-containing antacids | ||
| Darunavir, 800 mg (Prezista), once daily | Darunavir can cause rash (rarely, Stevens-Johnson syndrome), diarrhea, hepatotoxicity, hyperlipidemia, hyperglycemia, and fat maldistribution; use with caution in patients with severe sulfa allergy | |
| plus | Highest risk of drug interactions; other regimens may be preferred for patients with certain comorbidities (e.g., dyslipidemia, diabetes mellitus) and those receiving long-term therapy with medications that lead to increased risk of drug interactions | |
| Ritonavir, 100 mg (Norvir), once daily | ||
| plus either | Tenofovir disoproxil fumarate use increases risk of nephrotoxicity and decreased bone mineral density; consider alternative (e.g., tenofovir alafenamide) in patients with or at risk of renal disease or osteopenia/osteoporosis; use with caution in patients who recently took or are taking nephrotoxic agents (e.g., nonsteroidal anti-inflammatory drugs) | |
| Coformulated emtricitabine, 200 mg/tenofovir disoproxil fumarate, 300 mg (Truvada), once daily | Truvada dosing should be adjusted or use should be reconsidered if creatinine clearance < 50 mL per minute per 1.73 m2; Truvada is not recommended if creatinine clearance < 30 mL per minute per 1.73 m2 | |
| or | Do not use Descovy in patients with creatinine clearance < 30 mL per minute per 1.73 m2 or in those receiving hemodialysis | |
| Coformulated emtricitabine, 200 mg/tenofovir alafenamide, 25 mg (Descovy), once daily | Lower risk of bone mineral loss and renal dysfunction with tenofovir alafenamide vs. tenofovir disoproxil fumarate, but possibly less favorable effects on lipid levels | |
| Patients with hepatitis B virus coinfection may be at increased risk of hepatitis flare when Truvada or Descovy is discontinued | ||
| Coformulated darunavir/cobicistat (Prezcobix) appears equivalent to ritonavir-boosted darunavir and can be administered once daily with food; Prezcobix should not be administered with tenofovir disoproxil fumarate–containing agents if creatinine clearance < 70 mL per minute per 1.73 m2 | ||
| Total daily pill count = 3; darunavir must be taken with food; should not be taken with magnesium-, aluminum-, or calcium-containing antacids | ||
| Dolutegravir, 50 mg (Tivicay), once daily | Tenofovir disoproxil fumarate use increases risk of nephrotoxicity and decreased bone mineral density; consider alternative (e.g., tenofovir alafenamide) in patients with or at risk of renal disease or osteopenia/osteoporosis; use with caution in patients who recently took or are taking nephrotoxic agents (e.g., nonsteroidal anti-inflammatory drugs) | |
| plus either | ||
| Coformulated emtricitabine, 200 mg/tenofovir disoproxil fumarate, 300 mg (Truvada), once daily | ||
| Truvada dosing should be adjusted or use should be reconsidered if creatinine clearance < 50 mL per minute per 1.73 m2; Truvada is not recommended if creatinine clearance < 30 mL per minute per 1.73 m2 | ||
| Do not use Descovy in patients with creatinine clearance < 30 mL per minute per 1.73 m2 or in those receiving hemodialysis | ||
| or | Lower risk of bone mineral loss and renal dysfunction with tenofovir alafenamide vs. tenofovir disoproxil fumarate, but possibly less favorable effects on lipid levels | |
| Coformulated emtricitabine, 200 mg/tenofovir alafenamide, 25 mg (Descovy), once daily | ||
| Dolutegravir inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting actual renal function | ||
| Multiple dolutegravir-associated drug interactions; overall lower risk of interactions than protease inhibitor– and cobicistat-containing regimens | ||
| Patients with hepatitis B or C virus coinfection may be at increased risk of transaminase elevation with dolutegravir use | ||
| Patients with hepatitis B virus coinfection may be at increased risk of hepatitis flare when Truvada or Descovy is discontinued | ||
| Total daily pill count = 2; no food restrictions; should not be taken with magnesium-, aluminum-, or calcium-containing antacids | ||
| Raltegravir, 400 mg (Isentress), twice daily | Raltegravir use can increase creatinine phosphokinase levels and risk of muscle pain/weakness | |
| Some raltegravir-associated drug interactions, but fewer than other integrase- and protease inhibitor– containing regimens | ||
| plus either | ||
| Coformulated emtricitabine, 200 mg/tenofovir disoproxil fumarate, 300 mg (Truvada), once daily | Tenofovir disoproxil fumarate use increases risk of nephrotoxicity and decreased bone mineral density; consider alternative (e.g., tenofovir alafenamide) in patients with or at risk of renal disease or osteopenia/osteoporosis; use with caution in patients who recently took or are taking nephrotoxic agents (e.g., nonsteroidal anti-inflammatory drugs) | |
| Truvada dosing should be adjusted or use should be reconsidered if creatinine clearance < 50 mL per minute per 1.73 m2; Truvada is not recommended if creatinine clearance < 30 mL per minute per 1.73 m2 | ||
| or | ||
| Coformulated emtricitabine, 200 mg/tenofovir alafenamide, 25 mg (Descovy), once daily | Lower risk of bone mineral loss and renal dysfunction with tenofovir alafenamide vs. tenofovir disoproxil fumarate, but possibly less favorable effects on lipid levels | |
| Patients with hepatitis B virus coinfection may be at increased risk of hepatitis flare when Truvada or Descovy is discontinued | ||
| Raltegravir is relatively well tolerated and lipid- and glucose-neutral | ||
| Total daily pill count = 3; no food restrictions; should not be taken with magnesium-, aluminum-, or calcium-containing antacids | ||
Response to ART is monitored by sequential measurement of viral load and CD4 counts.6 Optimal viral suppression is indicated by a viral load below the level of detection (usually less than 20 to 75 copies per mL, depending on the assay). Undetectable levels are typically achieved in eight to 24 weeks. Failure to achieve these thresholds should prompt assessment of medication adherence and problems of drug absorption or interactions; consultation with an HIV expert is encouraged. Table 1 provides the recommended intervals for laboratory monitoring for adverse effects of ART.6,12–15 Occasional short-term increases from undetectable or low viral loads can occur. Although these may not meet the criteria for virologic failure,6 persistence of viremia at detectable levels can indicate evolving ART resistance and increases the likelihood of treatment failure. Assessing treatment response by CD4 count is not as helpful because CD4 response lags behind changes in viral load. CD4 counts generally increase by 50 to 150 cells per mm3 (0.05 × 109 per L to 0.15 × 109 per L) per year until a steady state is achieved.
Immune reconstitution inflammatory syndrome is an immune-mediated reaction occurring two weeks to three months (or rarely, years) after ART initiation. It is more common among patients who start treatment at lower CD4 counts. In persons with untreated HIV infection, the immune system is suppressed; with effective ART, immune system recovery (indicated by an increasing CD4 count) can produce responses to latent infections and malignancies.6 Presentation includes fever, lymphadenopathy, or symptoms typical of the infection or process involved. These symptoms can be difficult to distinguish from adverse reactions associated with ART. Management depends on the pathogen and clinical status. Approaches include treatment of the underlying infection, supportive care, close observation, and, rarely, temporary discontinuation of ART. Consultation with an HIV expert should be considered to confirm the diagnosis of immune reconstitution inflammatory syndrome and to develop management strategies.
Opportunistic Infections
Prophylaxis against specific opportunistic infections is indicated for patients with substantial immunosuppression (Table 4).14 With effective ART, immune function can be restored and prophylaxis can be discontinued. Opportunistic infections are rare when the immune system is preserved, but patients with signs or symptoms of an opportunistic infection should be thoroughly evaluated, which might require consultation and/or hospitalization.
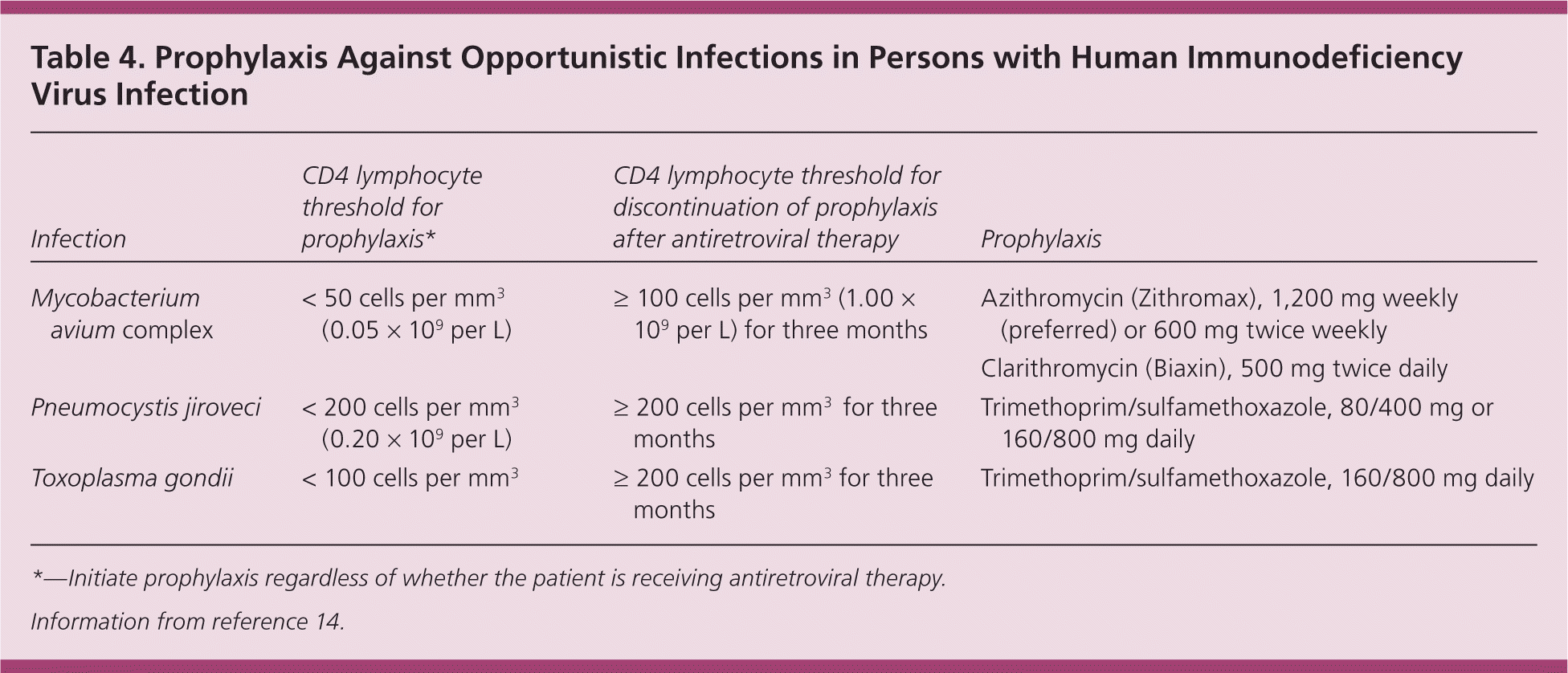
| Infection | CD4 lymphocyte threshold for prophylaxis* | CD4 lymphocyte threshold for discontinuation of prophylaxis after antiretroviral therapy | Prophylaxis |
|---|---|---|---|
| Mycobacterium avium complex | < 50 cells per mm3 (0.05 × 109 per L) | ≥ 100 cells per mm3(1.00 × 109 per L) for three months | Azithromycin (Zithromax), 1,200 mg weekly (preferred) or 600 mg twice weekly |
| Clarithromycin (Biaxin), 500 mg twice daily | |||
| Pneumocystis jiroveci | < 200 cells per mm3 (0.20 × 109 per L) | ≥ 200 cells per mm3 for three months | Trimethoprim/sulfamethoxazole, 80/400 mg or 160/800 mg daily |
| Toxoplasma gondii | < 100 cells per mm3 | ≥ 200 cells per mm3 for three months | Trimethoprim/sulfamethoxazole, 160/800 mg daily |
Psychosocial Aspects of HIV Primary Care
Assessment of the patient's social, family, and community support networks; financial situation; and housing can be critical. It is important to understand the patient's fears of stigma19 and disclosure; history of depression, anxiety, and suicidality; history of trauma or abuse, particularly at a young age (common among HIV-infected patients20); and any other psychosocial issues that strengthen the therapeutic relationship. Clinicians should determine how patients prefer their gender to be addressed and how this information is shared with family members or associates. Finally, it is helpful to understand the effect of the patient's HIV diagnosis on family members and associates.
Pregnant Women, Children, and Older Adults
Pregnant women who are HIV-positive should maintain continuous suppressive ART throughout pregnancy to reduce in utero infection and transmission at the time of delivery.15 With consistent perinatal treatment, mother-to-child transmission of HIV is rare. Consultation with experts or the Perinatal HIV Hotline (Table 5) should be sought. HIV infection in infants and children is generally managed in conjunction with pediatric HIV experts.

| AIDS Education and Training Center Program |
| http://aidsetc.org/ |
| AIDS Info (U.S. Dept. of Health and Human Services treatment guidelines) |
| https://aidsinfo.nih.gov/ |
| American Academy of HIV Medicine (referrals) |
| http://www.aahivm.org/ReferralLink/exec/frmAdvSearch.aspx |
| HIV Medicine Association |
| http://www.hivma.org/Home.aspx |
| HIV/AIDS Management Hotline (clinical advice on preventing and treating HIV) |
| 800-933-3413 (Monday through Friday, 9 a.m. to 8 p.m. EST) |
| Health Resources and Services Administration Ryan White Clinical Services |
| https://careacttarget.org/grants-map/all |
| International Antiviral Society–USA |
| https://www.iasusa.org |
| Perinatal HIV Hotline (advice on managing HIV in pregnancy, preventing perinatal transmission, and caring for infants exposed to HIV) |
| 888-448-8765 (24 hours, 7 days a week) |
| Postexposure Prophylaxis Hotline (guidance on managing occupational and nonoccupational exposures) |
| 888-448-4911 (9 a.m. to 2 a.m. EST, 7 days a week) |
| Preexposure Prophylaxis Hotline (guidance on indications, laboratory protocols, and treatment regimens) |
| 855-448-7737 (Monday through Friday, 11 a.m. to 6 p.m. EST) |
| University of California–San Francisco, Clinician Consultation Center: HIV/AIDS Management |
| http://nccc.ucsf.edu/ |
Older adults presenting for initial HIV care may include patients who have been infected recently and those with chronic infection who are newly diagnosed and entering or reentering care. Issues such as neurocognitive function, frailty, polypharmacy, medication interactions, multiple comorbidities, and social isolation are especially important in this population.21
Preventing Transmission
Persons with HIV should be counseled on measures to prevent transmission. All patients should be reminded of the usual safer sexual practices. Injection drug users should be advised of safer injection practices, community resources, and medication options for substance use.22 Clinicians should emphasize that ART resulting in viral load suppression can dramatically reduce transmission,7 but it does not eliminate the need for safer sexual and injection practices. When exposures to HIV do occur, prompt postexposure prophylaxis can reduce transmission.23 Preexposure prophylaxis is another important prevention tool in which uninfected partners at ongoing risk take antiretroviral medications daily to prevent infection. Consultation via a toll-free hotline is available (Table 5).
Partner notification is vitally important. Ideally, the patient would notify their current and recent partners, but this is often difficult. In relationships in which intimate partner violence is a possibility, notification can be unsafe. Primary care clinicians might need to enlist the help of their local health department to ensure proper partner notification.24
Consultation
Most components of the comprehensive care of persons with HIV-infection can be provided by primary care clinicians, but consultation or comanagement with an HIV expert is often needed.11,12,25 Cases of antiretroviral drug resistance, consideration of alternative regimens, and severe immunodeficiency are challenging and will often benefit from consultation with an HIV expert. Local and regional experts, often affiliated with universities, medical centers, and Ryan White–funded HIV clinics, are generally the first line of consultation. Links to local experts are listed in Table 5.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms HIV, AIDS, primary care, and guidelines. The search included meta-analyses, randomized controlled trials, clinical trials, guidelines, and reviews. We performed specific searches on key guidelines websites, including: http://www.aidsinfo.nih.gov; http://www.cdc.gov; and http://www.cdc.gov/mmwr, as well as the Cochrane database and https://www.aafp.org/journals/afp. Search dates: August 18 and 19, 2015; September 14 to 17, 2015; September 29 and 30, 2015; October 1 to 5, 2015; and September 9 and 10, 2016.
This article was made possible by AIDS Education and Training Center Program grant award #H4AHA01082 from the HIV/AIDS Bureau of the Health Resources and Services Administration, U.S. Dept. of Health and Human Services, with additional funding from the Centers for Disease Control and Prevention. The information presented is the responsibility of the authors and does not necessarily represent the official view of the Health Resources and Services Administration, the AIDS Education and Training Center Program, or the Centers for Disease Control and Prevention.