
A more recent article on autism spectrum disorder is available.
Am Fam Physician. 2016;94(12):972-979
Patient information: See related handout on autism spectrum disorder, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Autism spectrum disorder is characterized by difficulty with social communication and restricted, repetitive patterns of behavior, interest, or activities. The Diagnostic and Statistical Manual of Mental Disorders, 5th ed., created an umbrella diagnosis that includes several previously separate conditions: autistic disorder, Asperger syndrome, childhood disintegrative disorder, and pervasive developmental disorder not otherwise specified. There is insufficient evidence to recommend screening for autism spectrum disorder in children 18 to 30 months of age in whom the disorder is not suspected; however, there is a growing body of evidence that early intensive behavioral intervention based on applied behavior analysis improves cognitive ability, language, and adaptive skills. Therefore, early identification of autism spectrum disorder is important, and experts recommend the use of a validated screening tool at 18- and 24-month well-child visits. Medications can be used as adjunctive treatment for maladaptive behaviors and comorbid psychiatric conditions, but there is no single medical therapy that is effective for all symptoms of autism spectrum disorder. Prognosis is heavily affected by the severity of diagnosis and the presence of intellectual disability. Children with optimal outcomes receive earlier, more intensive behavioral interventions and less pharmacologic treatment.
Autism was first described by psychiatrist Leo Kanner in 1943 as a disorder in children who had problems relating to others and a high sensitivity to changes in their environment.1 Although it appeared to be a rare disorder at that time, the prevalence of autism spectrum disorder (ASD) steadily increased. The Centers for Disease Control and Prevention's (CDC's) monitored network of 11 locations has described an autism prevalence of one in 68 children, with a male-to-female ratio of 4.5-to-1.2 These data correlate with other studies across multiple nations and widely separated locations.3,4 The increase in ASD prevalence may be partially attributed to the evolving diagnostic criteria prior to the publication of Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5), an increase in social awareness, and mandatory availability of treatments. Additionally, school-aged children with higher functioning are being diagnosed with previously unrecognized ASD.5–7 In 2013, DSM-5 created the umbrella diagnosis of ASD, consolidating four previously separate disorders: autistic disorder, Asperger syndrome, childhood disintegrative disorder, and pervasive developmental disorder not otherwise specified.8
WHAT IS NEW ON THIS TOPIC: AUTISM SPECTRUM DISORDER
The U.S. Preventive Services Task Force concluded that current evidence is insufficient to assess the balance of benefits and harms of screening for autism spectrum disorder in young children for whom no concerns of autism spectrum disorder have been raised by their parents or a clinician.
In 2014, an Agency for Healthcare Research and Quality systematic review found a growing body of evidence that an applied behavior analysis–based early intensive behavioral intervention, delivered over an extended time frame, improves cognitive ability, language, and adaptive skills in autistic children.
More than 80% of patients with autism spectrum disorder retain the same level of severity on repeat assessment over an eight- to 10-year interval.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Screening for autism spectrum disorder with a validated tool is recommended at 18- and 24-month well-child visits to assist with early detection. | C | 33, 35 |
| In children with autism spectrum disorder, an applied behavior analysis–based early intensive behavioral intervention delivered over an extended time frame improves cognitive ability, language, and adaptive skills. | B | 39 |
| Cognitive behavior therapy is effective at lowering anxiety in older children with autism spectrum disorder who have an average or above-average IQ. | A | 39 |
| Melatonin helps manage sleep disorders, improves daytime behavior, and has minimal adverse effects in children with autism spectrum disorder. | A | 25, 26 |
Etiology
Studies of the genetic heritability of ASD range from 40% to 90%, with most recent estimates at nearly 50% genetic liability. The genetic contribution to ASD occurs via a diverse group of mutational mechanisms along many biologic pathways.9–12 Additional risk is associated with environmental factors. Prenatal risks include advanced paternal or maternal age and maternal metabolic conditions, such as diabetes mellitus, hypertension, and obesity.13 In utero risks include valproate (Depacon) exposure, maternal infections, traffic-related air pollution, and pesticide exposure.13 Perinatal events such as low birth weight and preterm delivery increase the risk of ASD as a part of the greater overall risk of neurodevelopmental injury.13 Previous concerns for causality related to thimerosal-based vaccines have been conclusively disproven. A summary of this evidence is available to review with concerned parents on the CDC's website at http://www.cdc.gov/vaccinesafety/concerns/autism.html.14
Clinical Presentation
Key diagnostic features of children with ASD include deficits in social communication and restricted, repetitive patterns of behavior, interest, or activities. The diagnostic criteria exist on a continuum of severity and functional impairment (Table 1 and Table 2).8 Some signs and symptoms may emerge between six and 12 months of age. In many cases, a reliable diagnosis can be made by 24 months of age.15–17 Social deficits and delays in spoken language are the most prominent features in children younger than three years. Joint attention is the ability to coordinate one's own attention between another person and a distant object to share interest. Neurotypical children respond to joint attention at 12 months of age and initiate it by 14 months of age. Those not demonstrating joint attention after 15 months of age should be evaluated for ASD. Parents may present with a concern for hearing loss because children with ASD may not respond after multiple attempts to get their attention by calling their name.18–21

| A. Persistent deficits in social communication and social interaction across multiple contexts, as manifested by the following, currently or by history (examples are illustrative, not exhaustive; see text): | |
| 1. Deficits in social-emotional reciprocity, ranging, for example, from abnormal social approach and failure of normal back-and-forth conversation; to reduced sharing of interests, emotions, or affect; to failure to initiate or respond to social interactions. | |
| 2. Deficits in nonverbal communicative behaviors used for social interaction, ranging, for example, from poorly integrated verbal and nonverbal communication; to abnormalities in eye contact and body language or deficits in understanding and use of gestures; to a total lack of facial expressions and nonverbal communication. | |
| 3. Deficits in developing, maintaining, and understanding relationships, ranging, for example, from difficulties adjusting behavior to suit various social contexts; to difficulties in sharing imaginative play or in making friends; to absence of interest in peers. | |
| Specify current severity: Severity is based on social communication impairments and restricted, repetitive patterns of behavior (see Table 2). | |
| B. Restricted, repetitive patterns of behavior, interests, or activities, as manifested by at least two of the following, currently or by history (examples are illustrative, not exhaustive; see text): | |
| 1. Stereotyped or repetitive motor movements, use of objects, or speech (e.g., simple motor stereotypies, lining up toys or flipping objects, echolalia, idiosyncratic phrases). | |
| 2. Insistence on sameness, inflexible adherence to routines, or ritualized patterns of verbal or nonverbal behavior (e.g., extreme distress at small changes, difficulties with transitions, rigid thinking patterns, greeting rituals, need to take same route or eat same food every day). | |
| 3. Highly restricted, fixated interests that are abnormal in intensity or focus (e.g., strong attachment to or preoccupation with unusual objects, excessively circumscribed or perseverative interests). | |
| 4. Hyper- or hyporeactivity to sensory input or unusual interest in sensory aspects of the environment (e.g., apparent indifference to pain/temperature, adverse response to specific sounds or textures, excessive smelling or touching of objects, visual fascination with lights or movement). | |
| Specify current severity: Severity is based on social communication impairments and restricted, repetitive patterns of behavior (see Table 2). | |
| C. Symptoms must be present in the early developmental period (but may not become fully manifest until social demands exceed limited capacities, or may be masked by learned strategies in later life). | |
| D. Symptoms cause clinically significant impairment in social, occupational, or other important areas of current functioning. | |
| E. These disturbances are not better explained by intellectual disability (intellectual developmental disorder) or global developmental delay. Intellectual disability and autism spectrum disorder frequently co-occur; to make comorbid diagnoses of autism spectrum disorder and intellectual disability, social communication should be below that expected for general developmental level. | |
| Note: Individuals with a well-established DSM-IV diagnosis of autistic disorder, Asperger's disorder, or pervasive developmental disorder not otherwise specified should be given the diagnosis of autism spectrum disorder. Individuals who have marked deficits in social communication, but whose symptoms do not otherwise meet criteria for autism spectrum disorder, should be evaluated for social (pragmatic) communication disorder. | |
| Specify if: | |
| With or without accompanying intellectual impairment | |
| With or without accompanying language impairment | |
| Associated with a known medical or genetic condition or environmental factor (Coding note: Use additional code to identify the associated medical or genetic condition.) | |
| Associated with another neurodevelopmental, mental, or behavioral disorder (Coding note: Use additional code[s] to identify the associated neurodevelopmental, mental, or behavioral disorder[s].) | |
| With catatonia (refer to the criteria for catatonia associated with another mental disorder, pp. 119–120, for definition) (Coding note: Use additional code 293.89 [F06.1] catatonia associated with autism spectrum disorder to indicate the presence of the comorbid catatonia.) | |
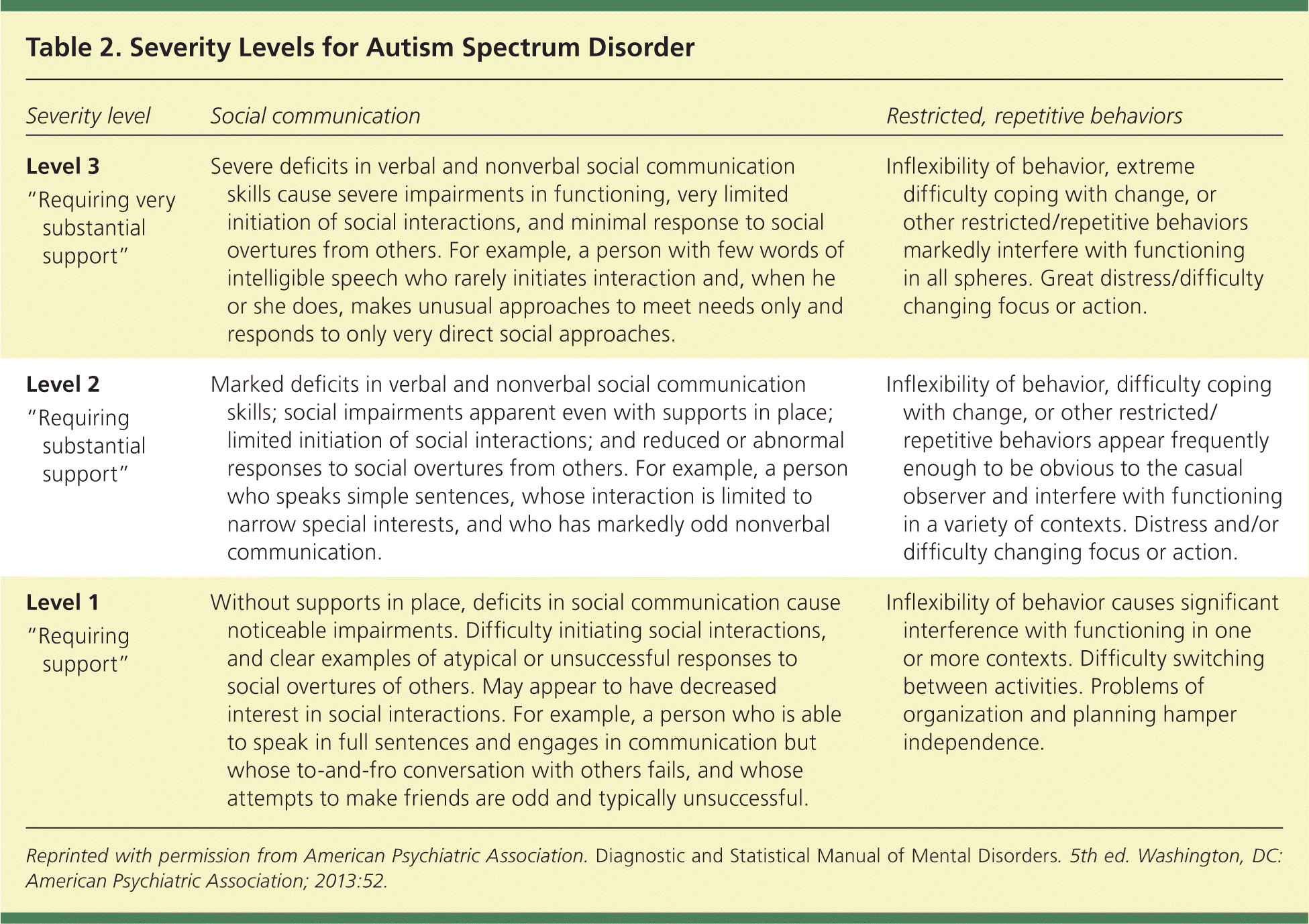
| Severity level | Social communication | Restricted, repetitive behaviors |
|---|---|---|
| Level 3 | Severe deficits in verbal and nonverbal social communication skills cause severe impairments in functioning, very limited initiation of social interactions, and minimal response to social overtures from others. For example, a person with few words of intelligible speech who rarely initiates interaction and, when he or she does, makes unusual approaches to meet needs only and responds to only very direct social approaches. | Inflexibility of behavior, extreme difficulty coping with change, or other restricted/repetitive behaviors markedly interfere with functioning in all spheres. Great distress/difficulty changing focus or action. |
| “Requiring very substantial support” | ||
| Level 2 | Marked deficits in verbal and nonverbal social communication skills; social impairments apparent even with supports in place; limited initiation of social interactions; and reduced or abnormal responses to social overtures from others. For example, a person who speaks simple sentences, whose interaction is limited to narrow special interests, and who has markedly odd nonverbal communication. | Inflexibility of behavior, difficulty coping with change, or other restricted/repetitive behaviors appear frequently enough to be obvious to the casual observer and interfere with functioning in a variety of contexts. Distress and/or difficulty changing focus or action. |
| “Requiring substantial support” | ||
| Level 1 | Without supports in place, deficits in social communication cause noticeable impairments. Difficulty initiating social interactions, and clear examples of atypical or unsuccessful responses to social overtures of others. May appear to have decreased interest in social interactions. For example, a person who is able to speak in full sentences and engages in communication but whose to-and-fro conversation with others fails, and whose attempts to make friends are odd and typically unsuccessful. | Inflexibility of behavior causes significant interference with functioning in one or more contexts. Difficulty switching between activities. Problems of organization and planning hamper independence. |
| “Requiring support” |
A restricted range of interest and repetitive behaviors are required for diagnosis of ASD. These may be less apparent in younger children and exist on a continuum. Change in routine is often a significant challenge for children with ASD. Unusual play patterns may be noted, such as focus on only part of a toy. Children with ASD may demonstrate stereotypic movements, such as hand flapping, toe walking, or finger flicking near their eyes18–21 (Table 318 ). A confounding variable for diagnosis is that children with ASD may have several coexisting conditions that impact the severity of the impairment (Table 42,18,22–29 ).
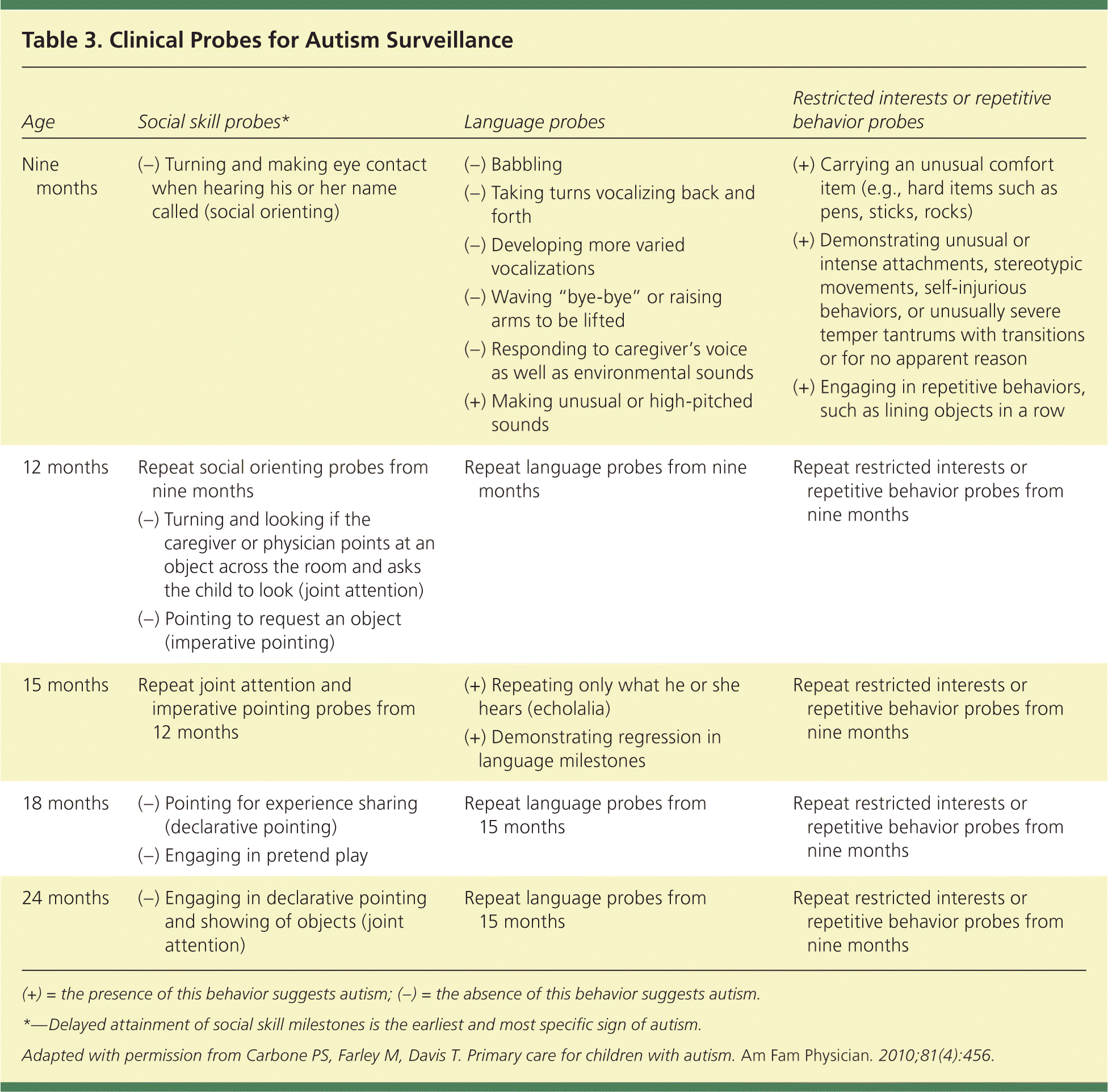
| Age | Social skill probes* | Language probes | Restricted interests or repetitive behavior probes |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
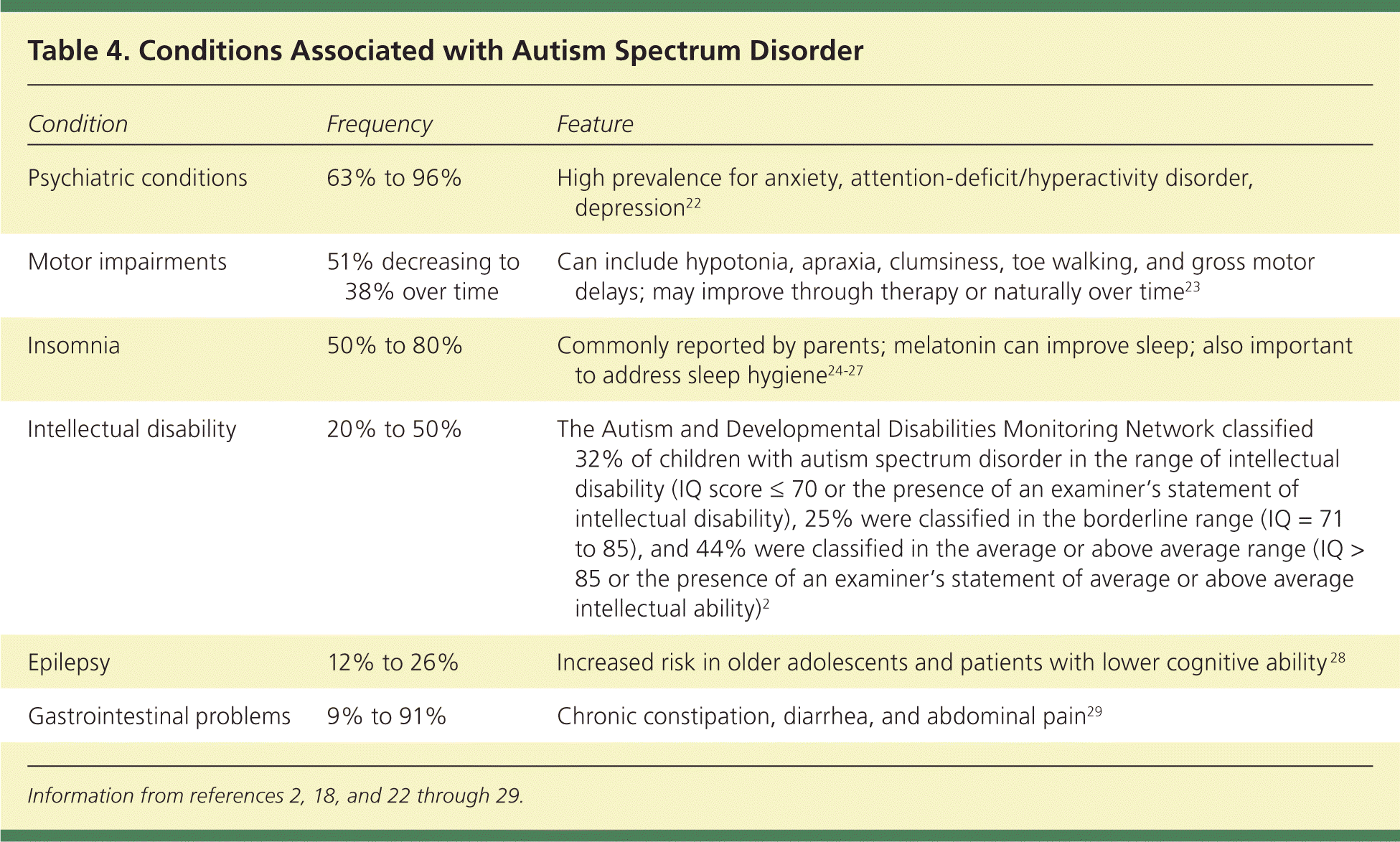
| Condition | Frequency | Feature |
|---|---|---|
| Psychiatric conditions | 63% to 96% | High prevalence for anxiety, attention-deficit/hyperactivity disorder, depression22 |
| Motor impairments | 51% decreasing to 38% over time | Can include hypotonia, apraxia, clumsiness, toe walking, and gross motor delays; may improve through therapy or naturally over time23 |
| Insomnia | 50% to 80% | Commonly reported by parents; melatonin can improve sleep; also important to address sleep hygiene24–27 |
| Intellectual disability | 20% to 50% | The Autism and Developmental Disabilities Monitoring Network classified 32% of children with autism spectrum disorder in the range of intellectual disability (IQ score ≤ 70 or the presence of an examiner's statement of intellectual disability), 25% were classified in the borderline range (IQ = 71 to 85), and 44% were classified in the average or above average range (IQ > 85 or the presence of an examiner's statement of average or above average intellectual ability)2 |
| Epilepsy | 12% to 26% | Increased risk in older adolescents and patients with lower cognitive ability 28 |
| Gastrointestinal problems | 9% to 91% | Chronic constipation, diarrhea, and abdominal pain29 |
Screening
Screening tools help identify children who may need a more thorough diagnostic assessment. Formal screening is more effective than relying on clinical judgment alone.30 However, there are no randomized clinical trials assessing the effectiveness of screening for ASD in children three years or younger based on long-term outcomes. The American Academy of Family Physicians and the U.S. Preventive Services Task Force found insufficient evidence to make a recommendation for screening in children 18 to 30 months of age in whom no concerns of ASD are suspected.31,32 Routine developmental screening is suggested at nine-, 18-, and 24- or 30-month well-child visits.33,34 The American Academy of Pediatrics recommends targeted screening for ASD with a validated screening tool at 18 and 24 months of age for early identification.33,35 The Modified Checklist for Autism in Toddlers (M-CHAT) is the most widely used screening tool. However, when used alone, it has poor positive predictive value and a high false-positive rate. The authors of that tool have since published the Modified Checklist for Autism in Toddlers–Revised, with Follow-Up (M-CHAT-R/F).36 The M-CHAT-R/F is a two-stage parent-reported screening tool to assess the risk of ASD. The M-CHAT-R/F may be downloaded free of charge for clinical, research, and educational purposes at http://mchatscreen.com/wp-content/uploads/2015/09/M-CHAT-R_F.pdf. A positive screening test result or parental concerns at any age should be followed by a structured interview and, if indicated, a referral for diagnostic assessment.33,35
Referral and Diagnosis
Evaluation for ASD should include a comprehensive assessment, preferably by an interdisciplinary team33,35 (eTable A). The evaluation aims to definitively diagnose ASD, exclude conditions that mimic ASD, identify comorbid conditions, and determine the child's level of functioning. In the absence of a team, an individual clinician with expertise in evaluating ASD (e.g., child psychologist, developmental pediatrician) is appropriate. The evaluation should include a complete history and direct assessment of social communication skills and restricted, repetitive behaviors using a semi-structured tool (e.g., the Autism Diagnostic Observation Schedule, 2nd ed.) with standardized testing of language and cognitive skills. The diagnosis must be confirmed using the DSM-5 criteria for ASD.33–36
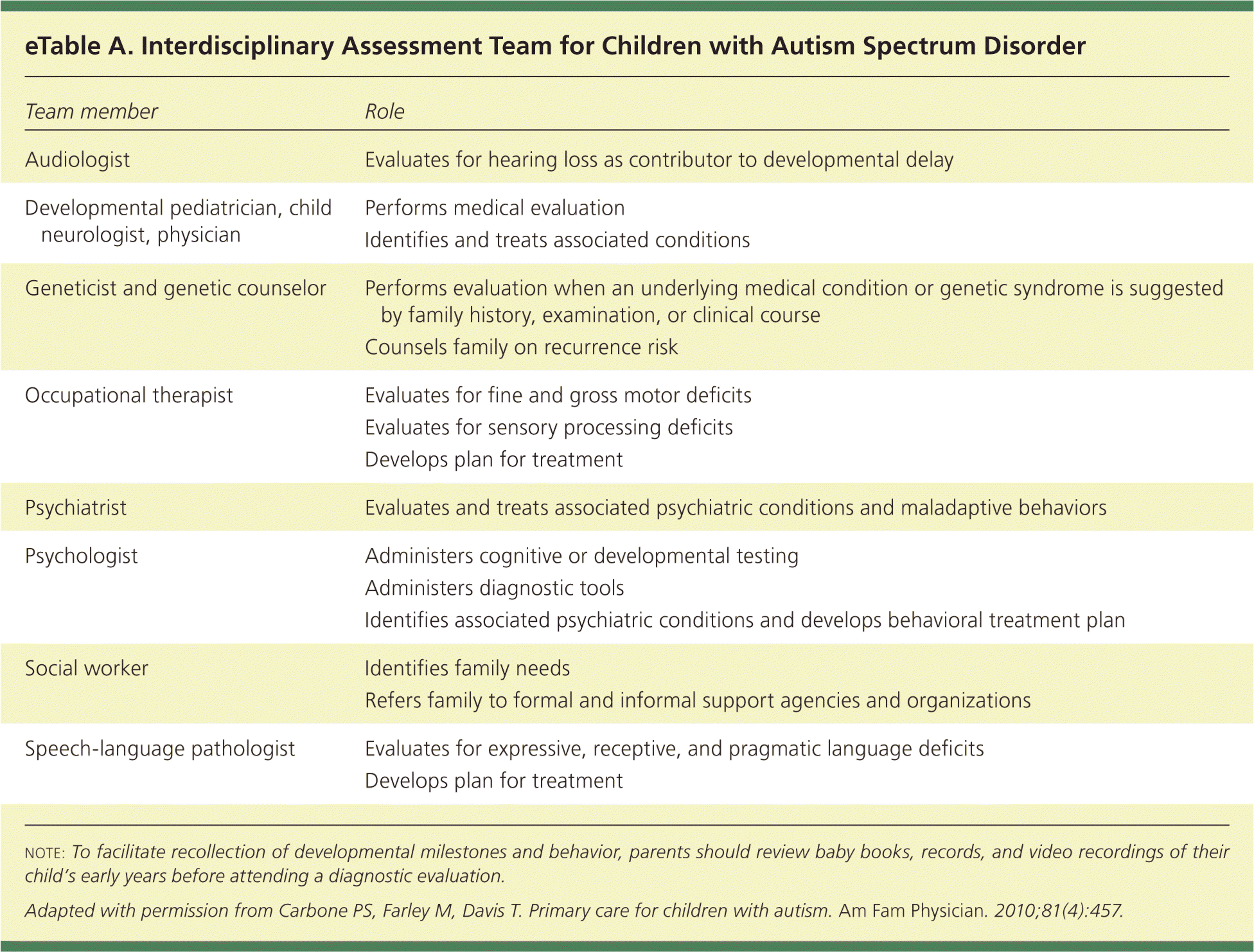
| Team member | Role |
|---|---|
| Audiologist | Evaluates for hearing loss as contributor to developmental delay |
| Developmental pediatrician, child neurologist, physician | Performs medical evaluation |
| Identifies and treats associated conditions | |
| Geneticist and genetic counselor | Performs evaluation when an underlying medical condition or genetic syndrome is suggested by family history, examination, or clinical course |
| Counsels family on recurrence risk | |
| Occupational therapist | Evaluates for fine and gross motor deficits |
| Evaluates for sensory processing deficits | |
| Develops plan for treatment | |
| Psychiatrist | Evaluates and treats associated psychiatric conditions and maladaptive behaviors |
| Psychologist | Administers cognitive or developmental testing |
| Administers diagnostic tools | |
| Identifies associated psychiatric conditions and develops behavioral treatment plan | |
| Social worker | Identifies family needs |
| Refers family to formal and informal support agencies and organizations | |
| Speech-language pathologist | Evaluates for expressive, receptive, and pragmatic language deficits |
| Develops plan for treatment |
Behavioral Treatments
Early intensive behavioral intervention is an immersive behavioral therapy for at least 25 hours per week that is recommended for preschool- to early school–aged children with ASD.37 Applied behavior analysis is a cornerstone of most early intensive behavioral intervention approaches. It seeks to teach new skills by reinforcing desirable behaviors, encouraging generalization of these skills, and decreasing undesirable behaviors. In a landmark study published in 1987 based on the principles of applied behavior analysis, one-half of the patients assigned to treatment were able to be placed in a neurotypical classroom and complete first grade.38 In 2014, the Agency for Healthcare Research and Quality updated a systematic review of existing and new randomized clinical trials and cohort studies. This rigorous review found a growing body of evidence that an applied behavior analysis–based early intensive behavioral intervention delivered over an extended time frame leads to improvement in cognitive ability, language, and adaptive skills.39 These effects were clinically and statistically significant.
Strong evidence shows that cognitive behavior therapy substantially reduces anxiety symptoms in older children with ASD who have average to above-average IQ.39 Several other behavioral interventions have also been examined but have limited evidence of benefit. Targeted play has led to improvements in early social communication skills.39 Social skills training has demonstrated short-term improvement in social skills and emotional recognition in school-aged children without intellectual dysfunction.39 Parent training and education programs improve language skills and decrease disruptive behavior in children.39,40
Medical Management
Although there is no medication available to treat the composite symptoms of ASD, medical management can be a beneficial adjunct. Medical treatment targets specific maladaptive behaviors for which intensive behavioral therapy has not been effective. Medical management may also target comorbid diagnoses, such as anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), and sleep disorders. Underlying conditions such as headaches, sinusitis, and gastrointestinal disorders can mimic or increase behavior symptoms common to ASD. These conditions should be ruled out before initiating targeted therapy.18,41
Aripiprazole (Abilify) and risperidone (Risperdal) are the only medications approved by the U.S. Food and Drug Administration for the treatment of ASD. These atypical antipsychotics are approved for ASD-associated irritability and, in some trials, have proven beneficial for treating aggression, explosive outbursts, and self-injury.42,43 Aripiprazole is approved for children six to 17 years of age.42 Risperidone is approved for children five to 16 years of age.43 Although these medications may provide some benefits, they must be weighed against serious potential adverse effects including sedation, weight gain, tremor, and extrapyramidal symptoms. Subspecialty referral should be strongly considered for these treatments.
Stimulants such as methylphenidate (Ritalin) may prove beneficial in children with comorbid ADHD, but treatment effects are less significant than in children without ASD and adverse effects are more common. Non–stimulant-based treatments may have a larger role in children with comorbid ADHD and have shown fewer adverse effects.44
COMPLEMENTARY AND ALTERNATIVE TREATMENTS
Families of children with ASD are likely to try complementary and alternative treatments.24 A full list of treatments is beyond the scope of this article, but several therapies merit review. There is strong evidence that melatonin helps manage sleep disorders, improves daytime behavior, and has minimal adverse effects.24–26 Massage therapy has been studied in several single-blinded randomized controlled trials that demonstrated benefits on ASD symptoms, sleep, language, repetitive behaviors, and anxiety. Massage can be performed by parents and has no evidence of harm.24 A recent large randomized controlled trial of therapeutic horseback riding showed improvements in irritability and hyperactivity in children, with secondary outcomes of improved social communication and new word acquisition.45
Vitamin B6 and magnesium in larger doses have been studied for use in children with ASD to improve behavior, speech, and language. Results were equivocal, and at supratherapeutic doses, there is risk of neuropathy from vitamin B6 and diarrhea from magnesium toxicity.24,46 Additional treatments that are not recommended because they have not shown benefit across several randomized clinical trials include auditory integration training, facilitated communication, gluten- or casein-free diets, hyperbaric oxygen, and secretin.24,46,47
Prognosis
Outcome markers for adults with ASD include independent living, employment, friendship, and marriage. Early studies found that more than one-half of infants with autism were institutionalized. A high percentage of patients were described as having poor or very poor outcomes.48 Recent studies show slightly improved results. One limited study found that 12% of adults with ASD and an IQ of at least 70 lived independently.49
A 2012 study examined diagnostic stability as a marker of prognosis. Results showed that more than 80% of patients retain the same level of severity on repeat Autism Diagnostic Observation Schedule assessments over an eight- to 10-year interval, and only 15% were assigned to improving or worsening classes of ASD. Diagnostic severity and IQ levels were the best predictors of future function. The mildest class of ASD was dropped from this analysis, which left a bias toward more severe presentations.50
A small percentage of children with a documented history of ASD no longer meet diagnostic criteria and reach normal cognitive function. These children achieve an optimal outcome. When compared with a high-functioning ASD cohort, children with optimal outcomes had earlier referrals and more intensive interventions with more applied behavior analysis therapy and fewer pharmacologic interventions.51
Some articles have reframed the lens of rating scales by incorporating the patient's opinion as well as the parent's or caregiver's rating. These studies reflect a higher percentage of positive outcomes for patients with ASD based on the person-environment fit. Increasing daytime recreational activities and community inclusion improved the person-environment fit, resulting in higher levels of satisfaction. Additional studies that consider the entire autistic spectrum are needed to help clarify individual prognosis.48
Data Sources: Essential Evidence Plus was reviewed. References from the previous AFP article on ASD were reviewed. The Centers for Disease Control and Prevention website was reviewed for content and references. Additional searches were performed on PubMed, Ovid, and Google Scholar. Search terms included autism and autism spectrum disorder, combined with screening, prevalence, global prevalence, diagnosis, genetics, guidelines, ABA, behavioral therapy, risperidone, aripiprazole, selective serotonin reuptake inhibitor, tricyclic antidepressants, attention-deficit/hyperactivity disorder, ADHD, comorbidities, complementary and alternative medicine, B6, vitamin, melatonin, and prognosis. Search references were limited to 2010 and greater first; however, if limited options returned, the timeline was expanded to older references. Certain articles contained reviews of RCTs, and these references were also directly searched. Search dates: August to October 2015. Additional searches were made from March to April 2016 adding the terms severity and environment(al) risk factors.
The authors thank Michael Arnold, MD; Rob Lennon, MD, JD; and Danielle Sanchack, PsyD, for their support via review and edit of the text. They also thank Susan Ebbinghouse, medical librarian, for facilitating the literature search.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. government.
