
Am Fam Physician. 2016;94(12):980-986
Author disclosure: No relevant financial affiliations.
Gastrointestinal disorders are common complications of diabetes mellitus and include gastroparesis, nonalcoholic fatty liver disease, gastroesophageal reflux disease, and chronic diarrhea. Symptoms of gastroparesis include early satiety, postprandial fullness, nausea, vomiting of undigested food, bloating, and abdominal pain. Gastroparesis is diagnosed based on clinical symptoms and a delay in gastric emptying in the absence of mechanical obstruction. Gastric emptying scintigraphy is the preferred diagnostic test. Treatment involves glucose control, dietary changes, and prokinetic medications when needed. Nonalcoholic fatty liver disease and its more severe variant, nonalcoholic steatohepatitis, are becoming increasingly prevalent in persons with diabetes. Screening for nonalcoholic fatty liver disease is not recommended, and most cases are diagnosed when steatosis is found incidentally on imaging or from liver function testing followed by diagnostic ultrasonography. Liver biopsy is the preferred diagnostic test for nonalcoholic steatohepatitis. Clinical scoring systems are being developed that, when used in conjunction with less invasive imaging, can more accurately predict which patients have severe fibrosis requiring biopsy. Treatment of nonalcoholic fatty liver disease involves weight loss and improved glycemic control; no medications have been approved for treatment of this condition. Diabetes is also a risk factor for gastroesophageal reflux disease. Patients may be asymptomatic or present with atypical symptoms, including globus sensation and dysphagia. Diabetes also may exacerbate hepatitis C and pancreatitis, resulting in more severe complications. Glycemic control improves or reverses most gastrointestinal complications of diabetes.
Gastrointestinal (GI) disorders are common complications of diabetes mellitus. These complications typically involve dysmotility as a result of hyperglycemia.1 GI symptoms of diabetes are typically not related to the duration or type of diabetes, but may be influenced by the degree of glycemic control maintained by the patient.1 Additionally, these symptoms may be blunted in these patients because of neuropathy.2 GI symptoms of diabetes tend to be preventable, controllable, and sometimes reversible with tighter glycemic control.3
WHAT IS NEW ON THIS TOPIC: GASTROINTESTINAL COMPLICATIONS OF DIABETES MELLITUS
Patients with diabetes mellitus and gastroesophageal reflux disease are more likely to present with atypical symptoms, such as dysphagia or globus sensation. These patients are approximately twice as likely to develop esophageal dysplasia as those without diabetes.
Patients with diabetes and hepatitis C virus infection are more likely to develop severe liver-related complications, including fibrosis and hepatocellular carcinoma.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In patients with suspected diabetic gastroparesis, laboratory testing and imaging should be performed to rule out other possible causes of symptoms. | C | 5 |
| Gastric emptying scintigraphy after a solid meal is the preferred confirmatory test for gastroparesis. | C | 3, 5 |
| Metoclopramide (Reglan) is the first-line treatment for symptoms of gastroparesis. | B | 3, 5, 14 |
| Liver ultrasonography is more sensitive than hepatic function testing for the diagnosis of nonalcoholic fatty liver disease. | C | 19, 23 |
| Lifestyle modifications are the first-line treatment for nonalcoholic fatty liver disease. | C | 19, 23 |
| Limiting meal size may be effective for managing symptoms of diabetic esophageal dysmotility. | C | 3 |
Gastroparesis
Diabetic gastroparesis is a delay in the emptying of food from the upper GI tract in the absence of mechanical obstruction of the stomach or duodenum. Gastroparesis may be the most common GI complication of diabetes, occurring in approximately 5% of patients with type 1 diabetes and 1% of those with type 2 diabetes.4 Obesity in patients with type 2 diabetes increases the risk of gastroparesis.5
Symptoms of diabetic gastroparesis are caused by ineffective contractions of the corpus and antrum of the stomach, coupled with abnormal relaxation of the fundus and pylorus, resulting in diminished gastric capacity, diminished mixing of food contents in the stomach, and delayed gastric emptying.5 This abnormal functioning can lead to altered absorption of medications and destabilization of glycemic control due to mismatched postprandial glucose and insulin peaks. Early symptoms of gastroparesis may not be recognized by the physician or patient. Symptoms may begin with early satiety and postprandial fullness, then progress to nausea, vomiting of undigested food, bloating, and abdominal pain.5 Nausea is typically more severe after meals. Findings from the abdominal examination may be normal in patients with gastroparesis except for distension, epigastric tenderness, or diffuse tenderness.
EVALUATION
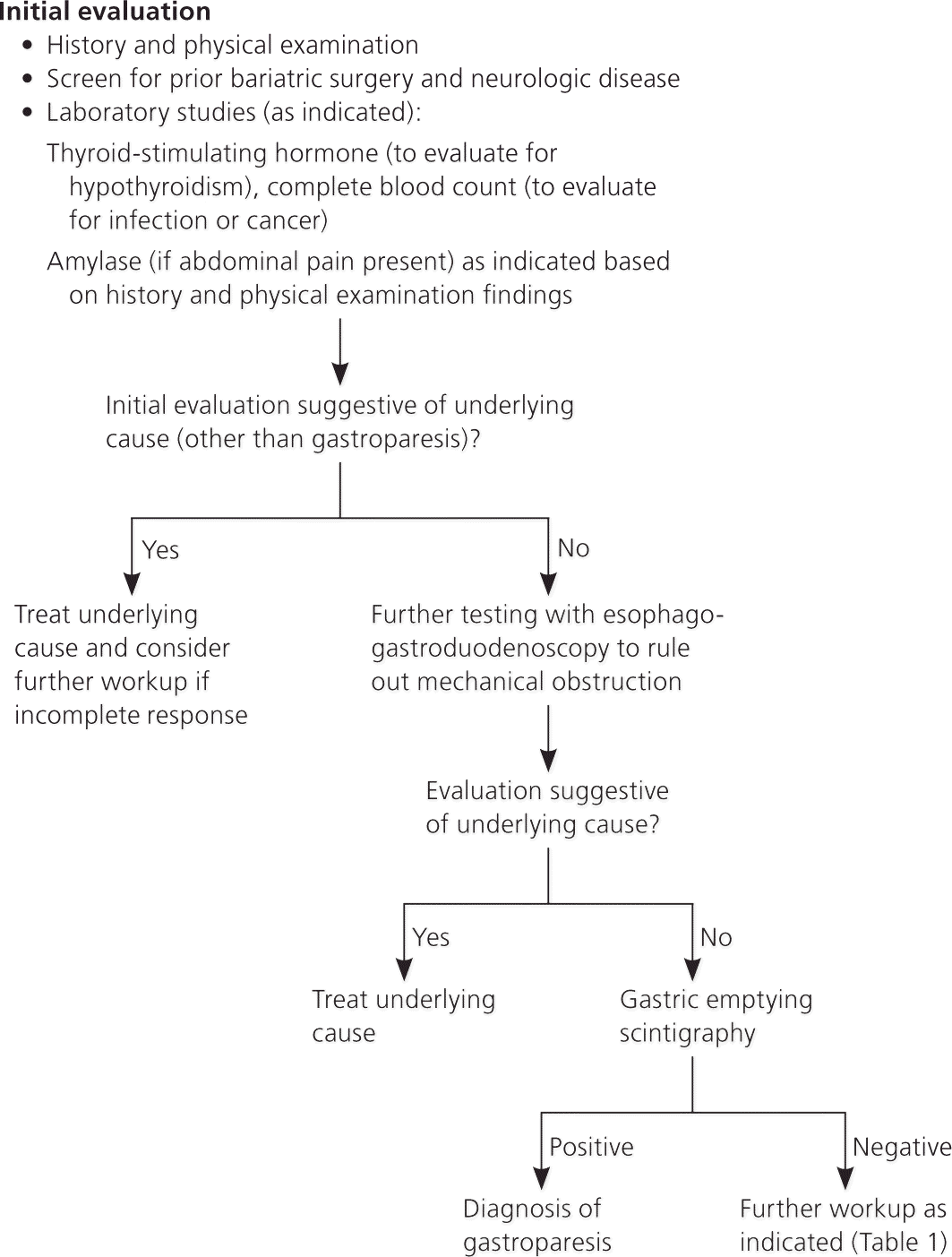
When gastroparesis secondary to diabetes is suspected, laboratory testing and imaging should be tailored to rule out other diagnoses suggested by the history and physical examination findings, such as hypothyroidism, infection, malignancies, pancreatitis, and neurologic disease.5 When other conditions have been ruled out, esophagogastroduodenoscopy is recommended to rule out sources of mechanical obstruction, in addition to testing to confirm gastric emptying. Gastric emptying scintigraphy after a solid meal is the preferred confirmatory test for gastroparesis3,5,6 (Table 15,7 and Figure 15 ).
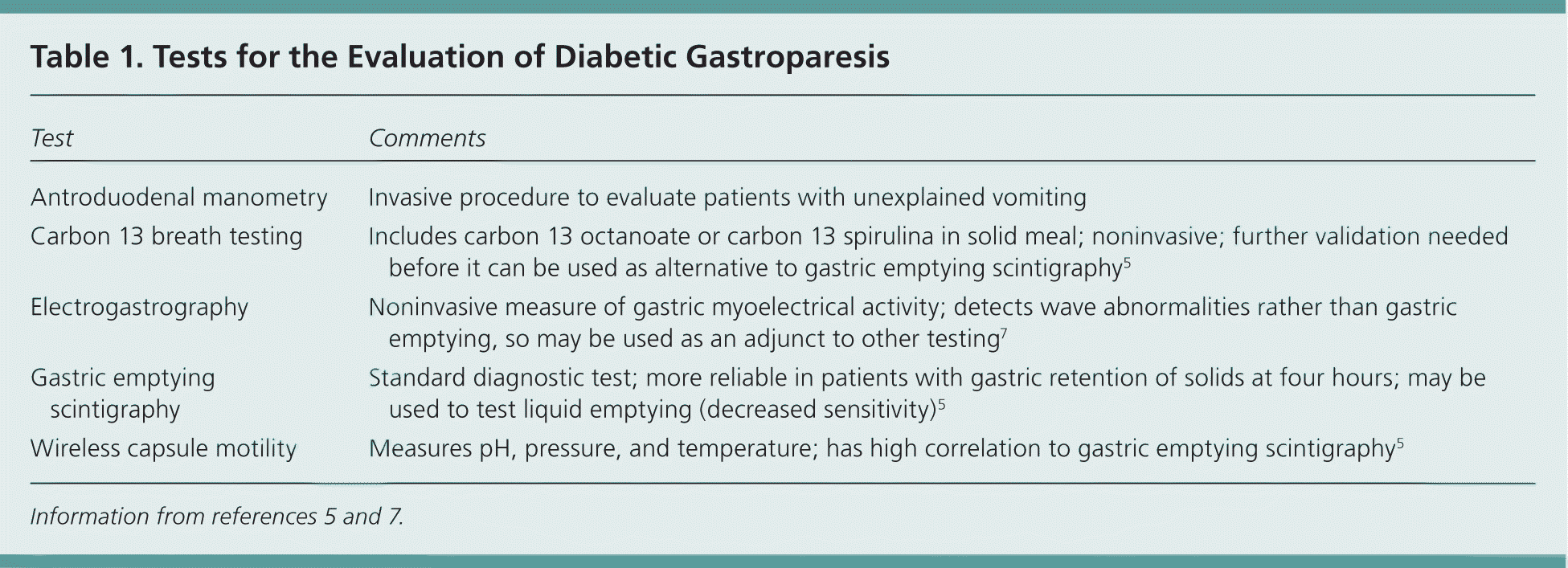
| Test | Comments |
|---|---|
| Antroduodenal manometry | Invasive procedure to evaluate patients with unexplained vomiting |
| Carbon 13 breath testing | Includes carbon 13 octanoate or carbon 13 spirulina in solid meal; noninvasive; further validation needed before it can be used as alternative to gastric emptying scintigraphy5 |
| Electrogastrography | Noninvasive measure of gastric myoelectrical activity; detects wave abnormalities rather than gastric emptying, so may be used as an adjunct to other testing7 |
| Gastric emptying scintigraphy | Standard diagnostic test; more reliable in patients with gastric retention of solids at four hours; may be used to test liquid emptying (decreased sensitivity)5 |
| Wireless capsule motility | Measures pH, pressure, and temperature; has high correlation to gastric emptying scintigraphy5 |
TREATMENT
Treatment for chronic gastroparesis begins with smaller, more frequent meals (as many as six to eight per day) and taking in more calories in semisolid or liquid form. Although liquid fats such as those in milk or nutritional supplements tend to be well tolerated, solid fats delay gastric emptying and exacerbate symptoms.5 Fiber also slows the rate of gastric emptying and can lead to formation of bezoars (solid masses of partially digested or undigested material that cause a blockage in the GI tract). Medications that reduce GI motility should be withheld when possible (Table 2).5,8–10 Patients with symptoms that are not controlled by diet alone can be treated with prokinetic medications and antiemetics for symptom relief (Table 3).5,11–15 Metoclopramide (Reglan) is the only prokinetic agent that has been studied specifically for gastroparesis and is considered first-line therapy.3,5,14
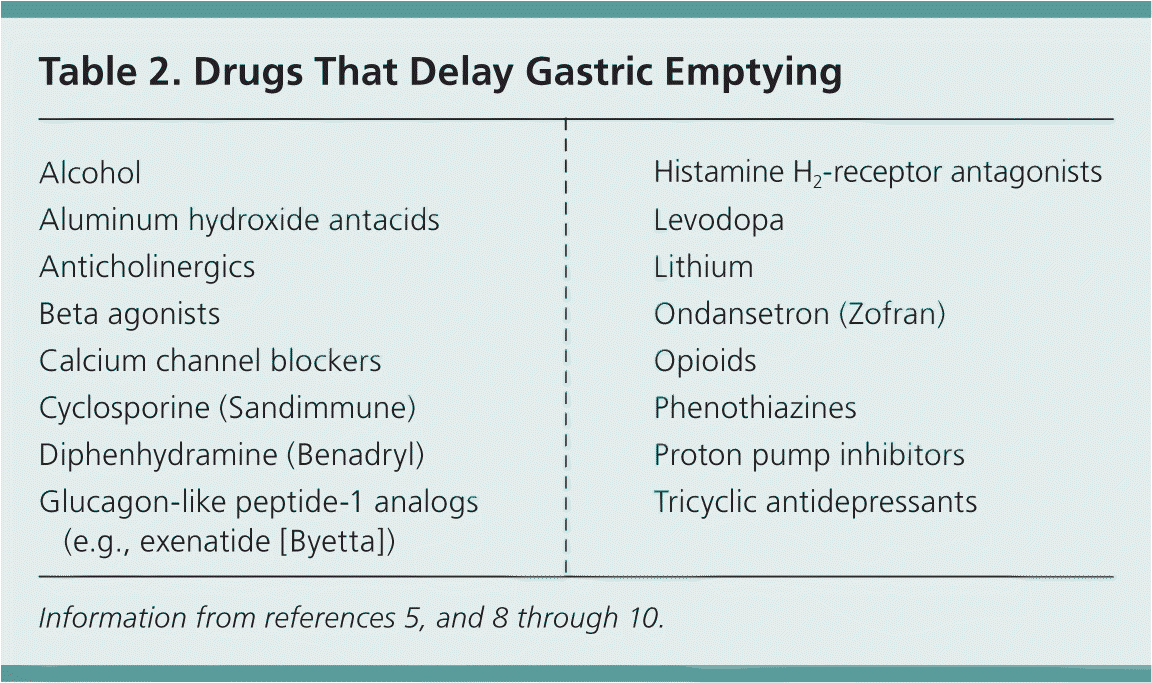
| Alcohol |
| Aluminum hydroxide antacids |
| Anticholinergics |
| Beta agonists |
| Calcium channel blockers |
| Cyclosporine (Sandimmune) |
| Diphenhydramine (Benadryl) |
| Glucagon-like peptide-1 analogs (e.g., exenatide [Byetta]) |
| Histamine H2-receptor antagonists |
| Levodopa |
| Lithium |
| Ondansetron (Zofran) |
| Opioids |
| Phenothiazines |
| Proton pump inhibitors |
| Tricyclic antidepressants |
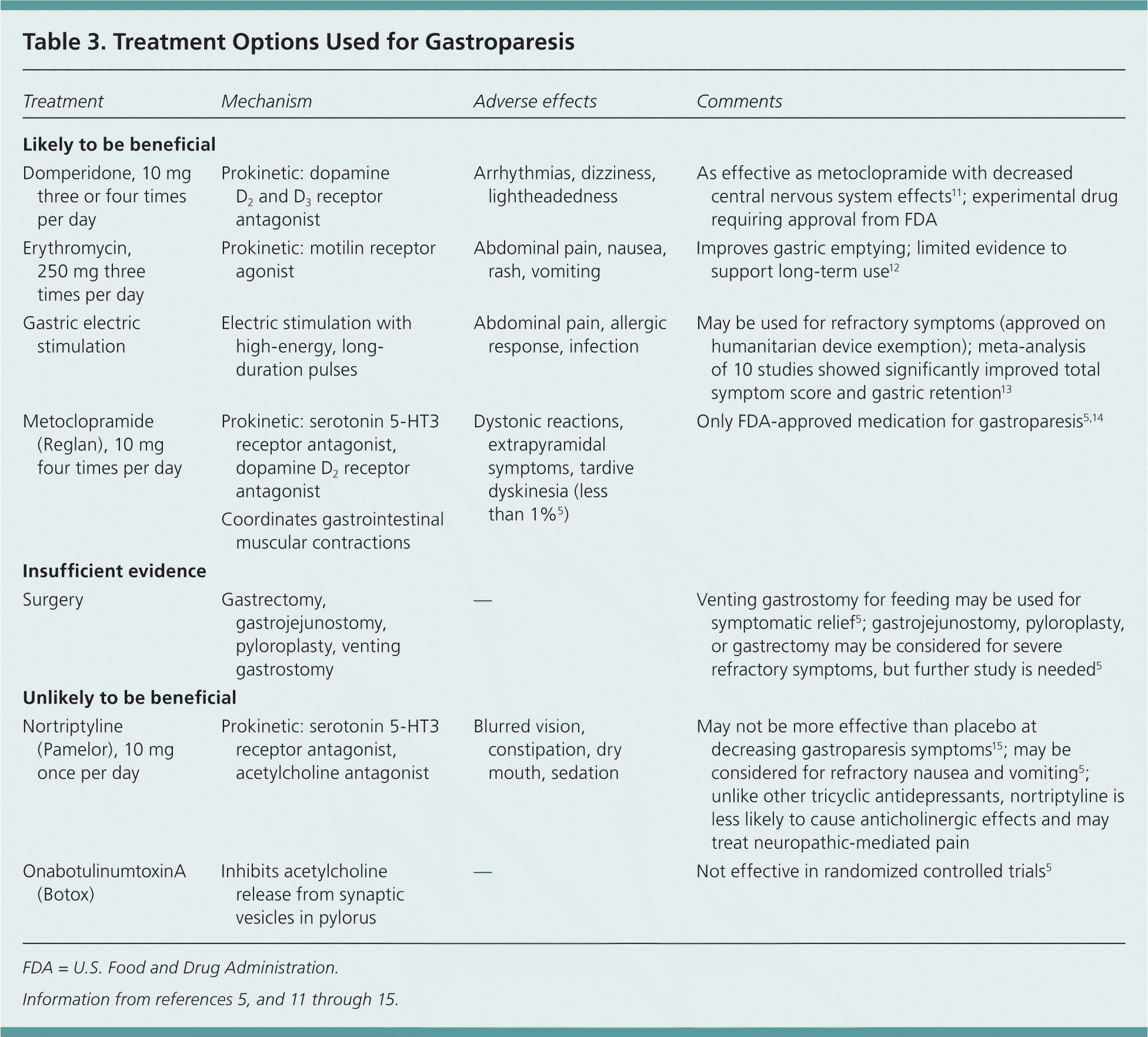
| Treatment | Mechanism | Adverse effects | Comments |
|---|---|---|---|
| Likely to be beneficial | |||
| Domperidone, 10 mg three or four times per day | Prokinetic: dopamine D2 and D3 receptor antagonist | Arrhythmias, dizziness, lightheadedness | As effective as metoclopramide with decreased central nervous system effects11; experimental drug requiring approval from FDA |
| Erythromycin, 250 mg three times per day | Prokinetic: motilin receptor agonist | Abdominal pain, nausea, rash, vomiting | Improves gastric emptying; limited evidence to support long-term use12 |
| Gastric electric stimulation | Electric stimulation with high-energy, long-duration pulses | Abdominal pain, allergic response, infection | May be used for refractory symptoms (approved on humanitarian device exemption); meta-analysis of 10 studies showed significantly improved total symptom score and gastric retention13 |
| Metoclopramide (Reglan), 10 mg four times per day | Prokinetic: serotonin 5-HT3 receptor antagonist, dopamine D2 receptor antagonist | Dystonic reactions, extrapyramidal symptoms, tardive dyskinesia (less than 1%5) | Only FDA-approved medication for gastroparesis5,14 |
| Coordinates gastrointestinal muscular contractions | |||
| Insufficient evidence | |||
| Surgery | Gastrectomy, gastrojejunostomy, pyloroplasty, venting gastrostomy | — | Venting gastrostomy for feeding may be used for symptomatic relief5; gastrojejunostomy, pyloroplasty, or gastrectomy may be considered for severe refractory symptoms, but further study is needed5 |
| Unlikely to be beneficial | |||
| Nortriptyline (Pamelor), 10 mg once per day | Prokinetic: serotonin 5-HT3 receptor antagonist, acetylcholine antagonist | Blurred vision, constipation, dry mouth, sedation | May not be more effective than placebo at decreasing gastroparesis symptoms15; may be considered for refractory nausea and vomiting5; unlike other tricyclic antidepressants, nortriptyline is less likely to cause anticholinergic effects and may treat neuropathic-mediated pain |
| OnabotulinumtoxinA (Botox) | Inhibits acetylcholine release from synaptic vesicles in pylorus | — | Not effective in randomized controlled trials5 |
In patients with chronic gastroparesis, symptom severity can be measured over time with the Gastroparesis Cardinal Symptom Index (Table 4).16 This is a validated instrument that can be used to track the progress of treatment.17 Additionally, the Abell scoring system provides treatment guidelines based on the severity of clinical signs and symptoms (Table 5).18
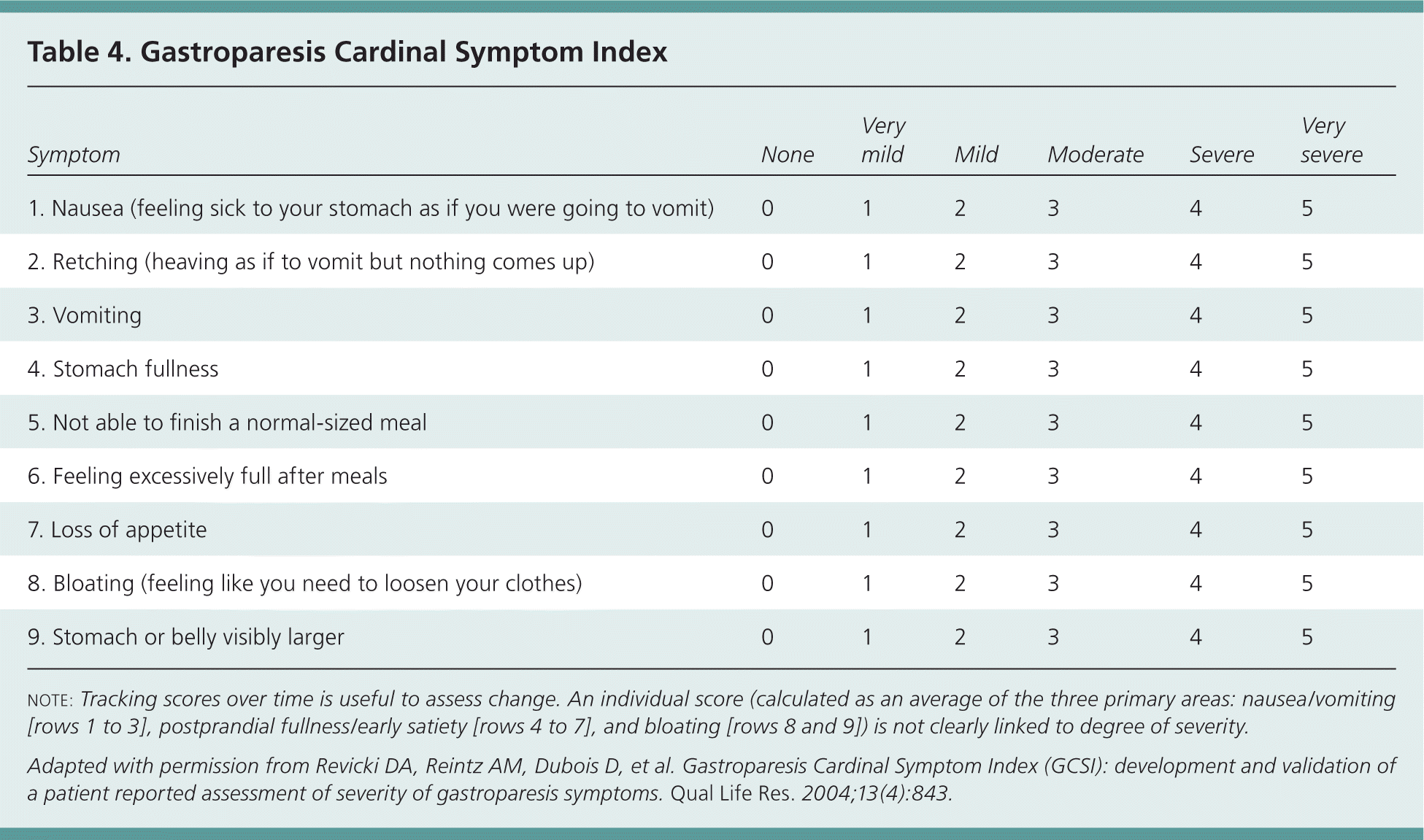
| Symptom | None | Very mild | Mild | Moderate | Severe | Very severe |
|---|---|---|---|---|---|---|
| 1. Nausea (feeling sick to your stomach as if you were going to vomit) | 0 | 1 | 2 | 3 | 4 | 5 |
| 2. Retching (heaving as if to vomit but nothing comes up) | 0 | 1 | 2 | 3 | 4 | 5 |
| 3. Vomiting | 0 | 1 | 2 | 3 | 4 | 5 |
| 4. Stomach fullness | 0 | 1 | 2 | 3 | 4 | 5 |
| 5. Not able to finish a normal-sized meal | 0 | 1 | 2 | 3 | 4 | 5 |
| 6. Feeling excessively full after meals | 0 | 1 | 2 | 3 | 4 | 5 |
| 7. Loss of appetite | 0 | 1 | 2 | 3 | 4 | 5 |
| 8. Bloating (feeling like you need to loosen your clothes) | 0 | 1 | 2 | 3 | 4 | 5 |
| 9. Stomach or belly visibly larger | 0 | 1 | 2 | 3 | 4 | 5 |
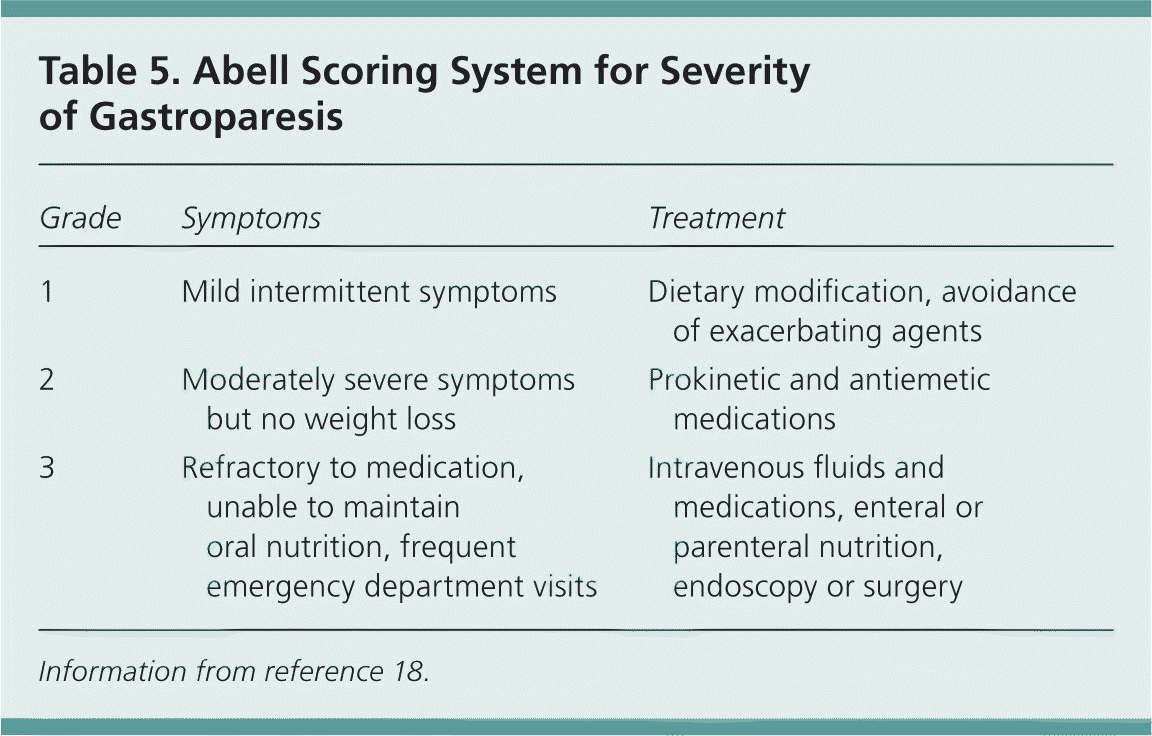
| Grade | Symptoms | Treatment |
|---|---|---|
| 1 | Mild intermittent symptoms | Dietary modification, avoidance of exacerbating agents |
| 2 | Moderately severe symptoms but no weight loss | Prokinetic and antiemetic medications |
| 3 | Refractory to medication, unable to maintain oral nutrition, frequent emergency department visits | Intravenous fluids and medications, enteral or parenteral nutrition, endoscopy or surgery |
Treatment of patients acutely ill with gastroparesis includes hydration and restoration of electrolyte imbalances, in addition to medications to control presenting symptoms such as nausea and vomiting.5 Hyperglycemia exacerbates symptoms of gastroparesis, so glucose control should be optimized, which may improve gastric emptying and reduce symptoms in the acute setting.3,5
Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of disease ranging from steatosis to severe fibrosis, referred to as nonalcoholic steatohepatitis (NASH).19 It is estimated that 30% of the U.S. population has NAFLD and 5% has NASH.20 These rates are thought to be even higher in those with diabetes or obesity; an estimated two-thirds of patients older than 50 years who have diabetes or obesity are thought to have NASH.19 Of those who develop NASH, 20% or more will develop cirrhosis, and 1% to 5% will develop hepatocellular carcinoma.19,21
Although the pathophysiology of NAFLD is unclear, insulin resistance is a major factor, resulting in increased free fatty acids and lipotoxicity.20 Because of their shared pathophysiology, NAFLD may be a complication of type 2 diabetes and vice versa.22 Having type 2 diabetes and NAFLD increases the risk of other diabetic complications (e.g., coronary artery disease, retinopathy) and may increase the risk of hepatic complications.22
The diagnosis of NAFLD includes hepatic steatosis (detected by imaging or histology) in the absence of secondary steatosis (e.g., caused by alcohol or medication use, or hereditary disorders). Screening is not recommended, and most patients are diagnosed based on abnormal liver function test results or incidental findings on imaging.23 Liver ultrasonography is more sensitive than hepatic function testing, which may have normal results in persons with NAFLD.23 Liver biopsy is the preferred diagnostic test, but it is limited to select high-risk patients because of the associated risks.23 Clinical scoring systems using laboratory data and demographic information are being used to quantify the risk of NASH to determine who may benefit from biopsy 24 (Table 623,25–28 ).
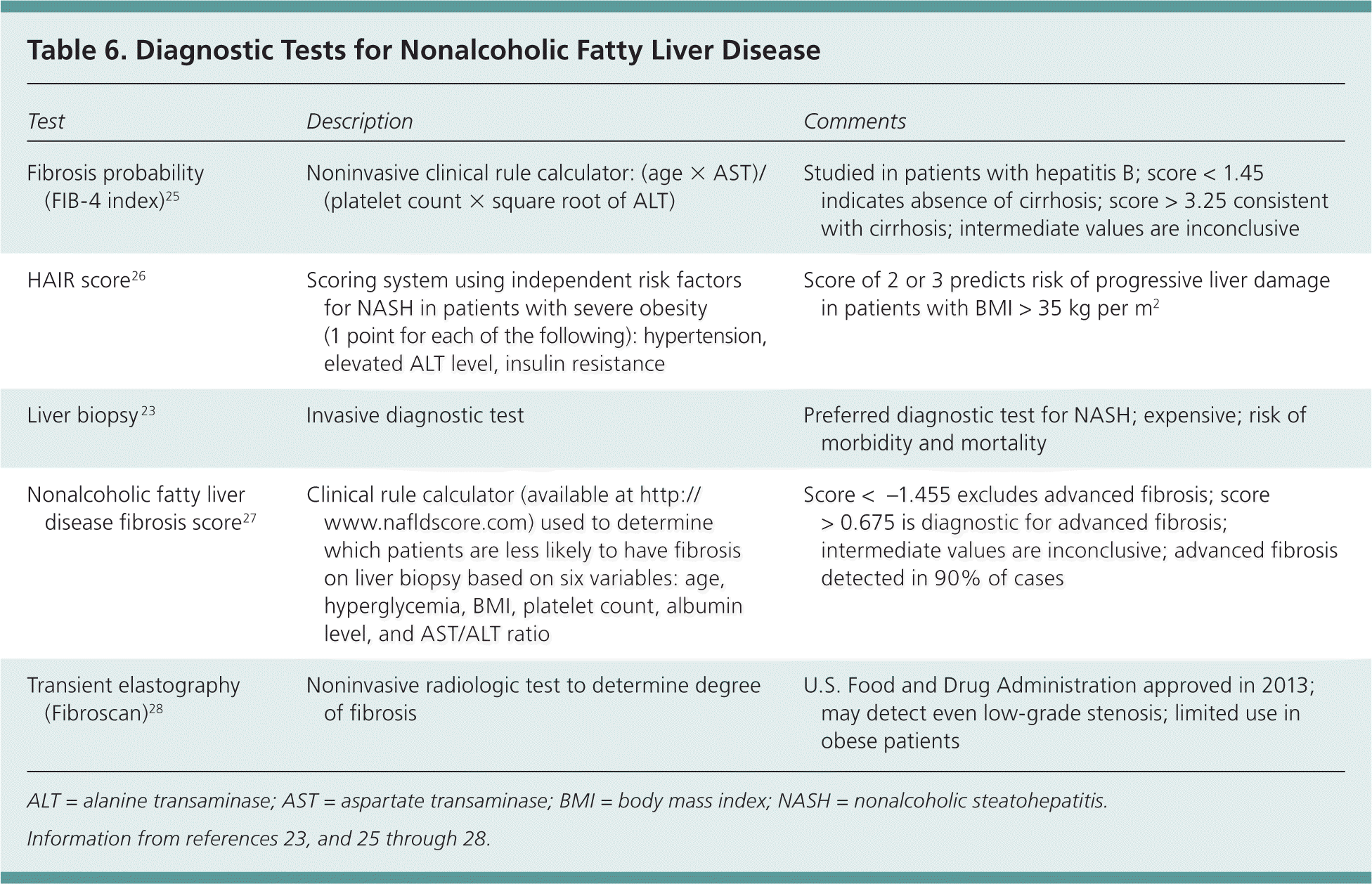
| Test | Description | Comments |
|---|---|---|
| Fibrosis probability (FIB-4 index)25 | Noninvasive clinical rule calculator: (age × AST)/(platelet count × square root of ALT) | Studied in patients with hepatitis B; score < 1.45 indicates absence of cirrhosis; score > 3.25 consistent with cirrhosis; intermediate values are inconclusive |
| HAIR score26 | Scoring system using independent risk factors for NASH in patients with severe obesity (1 point for each of the following): hypertension, elevated ALT level, insulin resistance | Score of 2 or 3 predicts risk of progressive liver damage in patients with BMI > 35 kg per m2 |
| Liver biopsy 23 | Invasive diagnostic test | Preferred diagnostic test for NASH; expensive; risk of morbidity and mortality |
| Nonalcoholic fatty liver disease fibrosis score27 | Clinical rule calculator (available at http://www.nafldscore.com) used to determine which patients are less likely to have fibrosis on liver biopsy based on six variables: age, hyperglycemia, BMI, platelet count, albumin level, and AST/ALT ratio | Score < −1.455 excludes advanced fibrosis; score > 0.675 is diagnostic for advanced fibrosis; intermediate values are inconclusive; advanced fibrosis detected in 90% of cases |
| Transient elastography (Fibroscan)28 | Noninvasive radiologic test to determine degree of fibrosis | U.S. Food and Drug Administration approved in 2013; may detect even low-grade stenosis; limited use in obese patients |
For those diagnosed with NAFLD, lifestyle modification is recommended as first-line therapy.19,23 Weight loss may decrease steatosis, although results are inconsistent.23 Bariatric surgery may support weight loss and potentially decrease steatosis, but it is not a recommended treatment.29 Several medications have been studied for the treatment of NAFLD, including pioglitazone (Actos), metformin, atorvastatin (Lipitor), and vitamin E, but none have been approved by the U.S. Food and Drug Administration for this indication.20
Esophageal Involvement
Esophageal dysmotility is linked to duration but not type of diabetes.30 It is thought to be multifactorial, resulting from the impact of neuropathy on motor and sensory function.31 Esophageal changes in patients with diabetes include reduced lower esophageal sphincter tone, prolonged transit time, and decreased coordination of esophageal contractions.31 Symptoms include dysphagia, odynophagia, heartburn, and chest pain, but some patients may be asymptomatic.3 Treatments for esophageal dysmotility are limited, although lifestyle modifications such as limiting meal size may be helpful.3
Diabetes is also a risk factor for gastroesophageal reflux disease, particularly for those with longer duration of diabetes and poor glycemic control.3 The diagnosis may be missed because patients with diabetes are more likely to be asymptomatic or present with atypical symptoms, such as dysphagia or globus sensation.32 In addition, patients with diabetes and gastroesophageal reflux disease are approximately twice as likely to develop dysplasia as those without diabetes.32 Lifestyle interventions to promote weight loss for adults with diabetes have been found to decrease gastroesophageal reflux symptoms such as heartburn and regurgitation.33
Intestinal Enteropathy
Intestinal enteropathy includes diabetic diarrhea, constipation, and fecal incontinence. The physiology contributing to enteropathy includes impairments in autonomic neuropathy that result in impaired small bowel motility, impaired electrolyte and fluid absorption, and abnormal resting tone of the internal and external sphincter.3 Diabetic diarrhea is more prevalent in patients with type 1 diabetes and should not be confused with iatrogenic diarrhea (e.g., from metformin use) in patients with type 2 diabetes.34 Persons with diabetes and chronic diarrhea are also at risk of anorectal dysfunction resulting in fecal incontinence, but this is less common.30
Association Between Diabetes and Other GI Diseases
DIABETES AND CELIAC DISEASE
Patients with type 1 diabetes have a five- to 10-fold higher prevalence of celiac disease, which may be related to common HLA genotypes.35 Screening for celiac disease in patients with type 1 diabetes is not currently recommended, but given its incidence, a lower threshold for screening may be appropriate.
DIABETES AND OTHER HEPATIC CONDITIONS
There is an epidemiologic link between hepatitis C and type 2 diabetes; infection with hepatitis C virus increases the risk of diabetes.36 Patients with diabetes and hepatitis C virus infection are more likely to develop severe liver-related complications, including fibrosis and hepatocellular carcinoma.37 In addition, diabetes confers an increased risk of hepatitis B because of the potential for shared lancets and needles; this risk contributed to the recommendation to vaccinate patients with diabetes for hepatitis B.38 Lastly, diabetic hepatopathy involves overloading of hepatocytes with glycogen in persons with poorly controlled type 1 diabetes. This condition may present similarly to acute hepatitis with abdominal pain and abnormal liver function test results.30
DIABETES AND PANCREATITIS/PANCREATIC CANCER
Diabetes is associated with an increased risk of pancreatitis and severe pancreatitis-related complications.39 Diabetes has also been linked with an increased risk of pancreatic cancer, particularly in patients recently diagnosed with diabetes.40 New-onset diabetes may be an early manifestation of pancreatic cancer as a result of damage to the pancreas. However, screening for pancreatic cancer in this population is not currently recommended.40
Data Sources: A PubMed search was completed in Clinical Queries using the search terms esophageal dysmotility and diabetes, NAFLD and diabetes, and gastroparesis. The search included meta-analyses, randomized controlled trials, case reports, and review articles. We also searched the Essential Evidence Plus summary report and Cochrane reviews. Search dates: December 15, 2016, and December 15, 2016.
