Am Fam Physician. 2017;95(1):online
Author disclosure: No relevant financial affiliations.

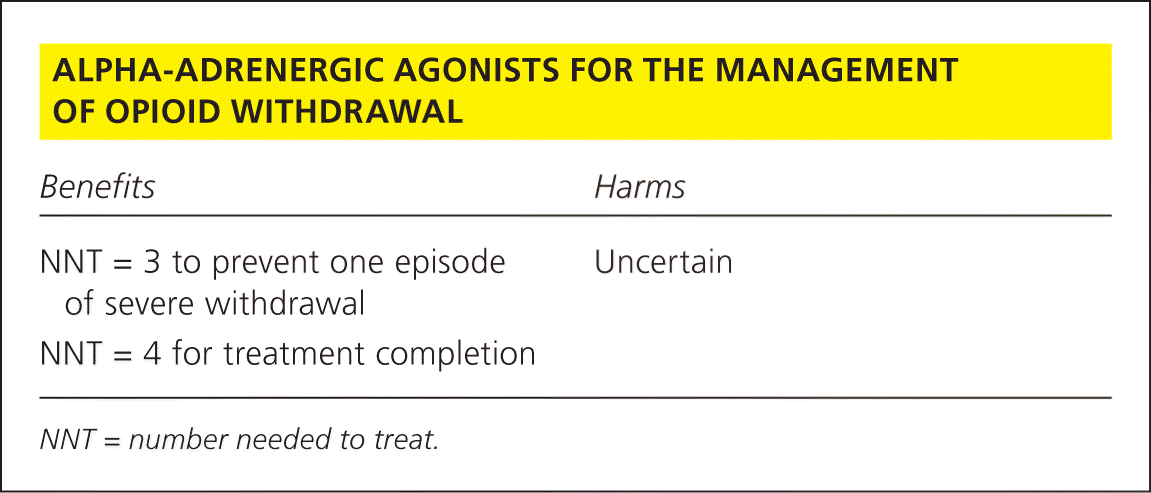
| Benefits | Harms |
|---|---|
| NNT = 3 to prevent one episode of severe withdrawal | Uncertain |
| NNT = 4 for treatment completion |
Details for This Review
Study Population: Patients undergoing managed withdrawal treatment for opioid dependence, spanning inpatient and outpatient settings
Efficacy End Points: Severe withdrawal symptoms (Modified Himmelsbach Opiate Withdrawal Scale; Objective Opioid Withdrawal Scale), treatment completion
Harm End Points: Hypotension, intense opioid cravings, uncomfortable symptoms—dizziness, drowsiness, fatigue, insomnia, and dry mouth
Narrative: Methadone is the most commonly used medication for management of withdrawal from opioids.1 Several factors (drug prescribing regulations, dislike for the prolonged nature of methadone withdrawal, and desire to find alternatives for opiate treatment) led to the investigation of alpha-adrenergic agonists (AAAs) to decrease the severity of withdrawal symptoms.2
A Cochrane review, including 26 randomized controlled trials totaling 1,728 participants, sought to compare AAAs vs. methadone and AAAs vs. placebo in effectively decreasing severe withdrawal symptoms as well as increasing the number of participants completing treatment.3 Investigators found moderate-quality evidence that AAAs were more effective than placebo in decreasing severe withdrawal symptoms with increased likelihood of treatment completion. Pooled analysis data of moderate-quality evidence revealed that 19% of the participants in the AAA group experienced severe withdrawal compared with 59% of those receiving placebo (number needed to treat [NNT] = 3; 95% confidence interval [CI], 2 to 4). Completion of treatment was 56% in those receiving AAAs vs. 33% in those receiving placebo (NNT = 4; 95% CI, 2 to 10).
Pooled analysis data as they relate to AAAs vs. methadone treatment were not as favorable. Rates of severe withdrawal were similar in both groups (24% for AAAs vs. 20% for methadone) and were not statistically significant. Rates of treatment completion were similar as well (48% for AAAs vs. 57% for methadone) and not statistically significant. Those in the AAA group appear to have experienced a greater incidence of hypotension or other adverse effects (15% for AAAs vs. 7.9% for methadone). However, when the studies at high risk of bias were removed from the analysis, the results were no longer statistically significant. Duration of treatment was shorter with AAAs vs. methadone.
Caveats: The quality of evidence for AAAs vs. decreasing doses of methadone was low due to three studies being at risk of selection bias and one study at risk of performance and detection bias. Significant heterogeneity was also present in the included studies. The quality of evidence for AAAs vs. placebo was moderate mainly because of the small number of events. It is likely that new evidence could change estimates of efficacy. Amongst the studies, there were various means of assessing withdrawal severity with multiple interventions compared simultaneously across inpatient and outpatient settings. Although overall duration of treatment was reported as 1.07 standard deviations shorter in the AAA group, the actual differences in duration are not explicitly given. Longer duration in the methadone group is likely related to the specific schedule of the methadone taper. It is unclear what effect duration has on long-term outcome in the treatment of opioid dependence. Retention in treatment may provide greater opportunity for psychosocial support as well as other aspects of care.4
Although AAAs were more efficacious than placebo in the management of opioid withdrawal, there was no significant difference in efficacy or completion rates between AAAs and methadone in the management of opioid withdrawal. Given the potential for adverse effects, AAAs should be reserved for select cases where an alternative to methadone is desired.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Contributing Editor, and Daniel Runde, MD, from the NNT Group (theNNT.com).
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/alpha-adrenergic-agonists-management-opioid-withdrawal/.