
Am Fam Physician. 2017;95(6):online
As published by the U.S. Preventive Services Task Force.
Summary of Recommendation and Evidence
The USPSTF recommends screening for latent tuberculosis infection (LTBI) in populations at increased risk (Table 1). B recommendation.
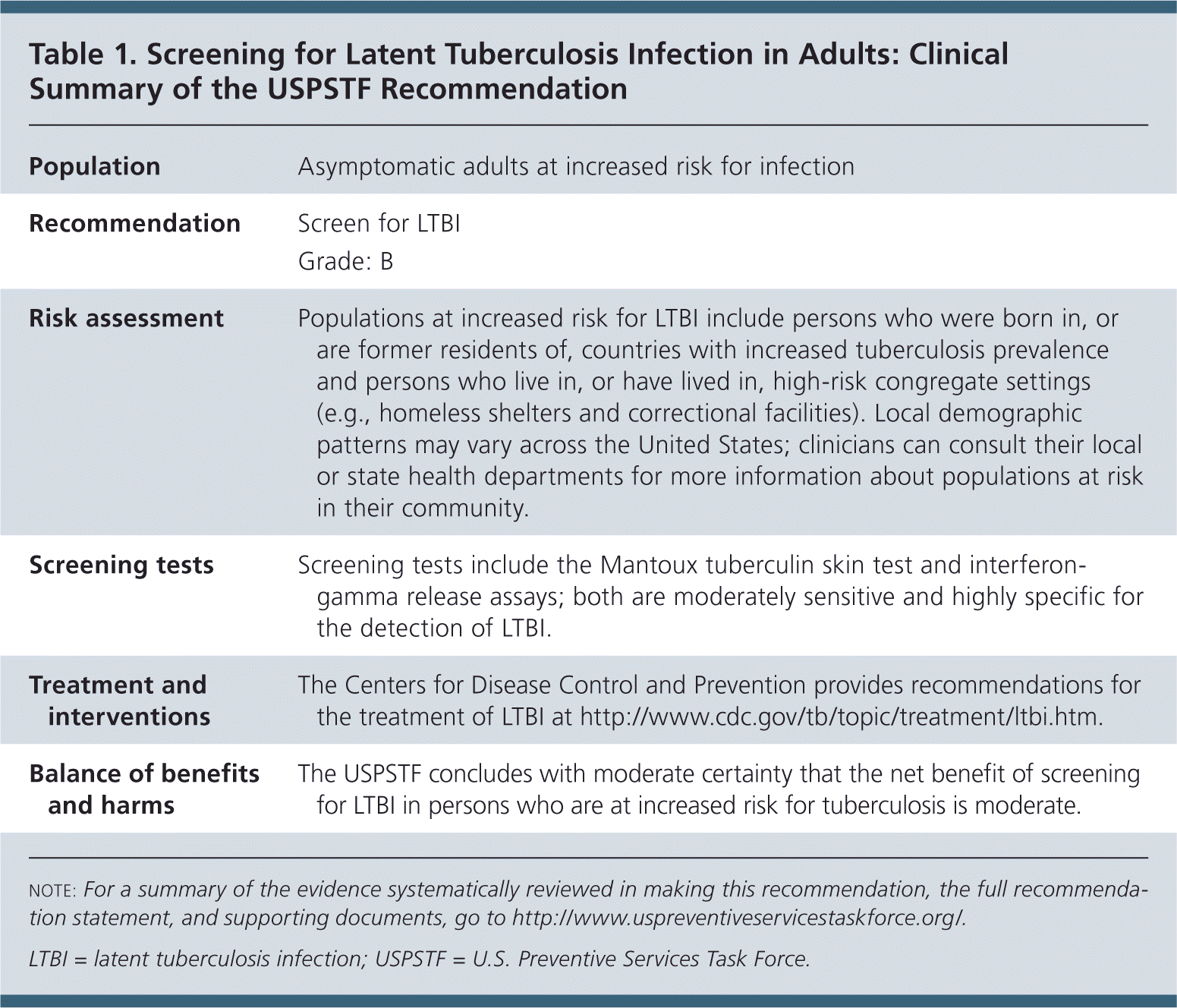
| Population | Asymptomatic adults at increased risk for infection |
| Recommendation | Screen for LTBI |
| Grade: B | |
| Risk assessment | Populations at increased risk for LTBI include persons who were born in, or are former residents of, countries with increased tuberculosis prevalence and persons who live in, or have lived in, high-risk congregate settings (e.g., homeless shelters and correctional facilities). Local demographic patterns may vary across the United States; clinicians can consult their local or state health departments for more information about populations at risk in their community. |
| Screening tests | Screening tests include the Mantoux tuberculin skin test and interferon-gamma release assays; both are moderately sensitive and highly specific for the detection of LTBI. |
| Treatment and interventions | The Centers for Disease Control and Prevention provides recommendations for the treatment of LTBI at http://www.cdc.gov/tb/topic/treatment/ltbi.htm. |
| Balance of benefits and harms | The USPSTF concludes with moderate certainty that the net benefit of screening for LTBI in persons who are at increased risk for tuberculosis is moderate. |
Rationale
IMPORTANCE
In the United States, tuberculosis remains an important preventable disease, including active tuberculosis, which may be infectious, and latent infection (LTBI), which is asymptomatic and not infectious but can later reactivate and progress to active disease. The precise prevalence rate of LTBI in the United States is difficult to determine; however, based on 2011–2012 National Health and Nutrition Examination Survey data, estimated prevalence is 4.7% to 5.0%.1 Tuberculosis is spread through respiratory transmission. Approximately 30% of persons exposed to Mycobacterium tuberculosis will develop LTBI and, if untreated, approximately 5% to 10% of these persons will progress to active tuberculosis disease or reactivation of tuberculosis.2–6 Rates of progression may be higher in persons with certain risk factors or medical conditions. An effective strategy for reducing the transmission, morbidity, and mortality of active tuberculosis disease is the identification and treatment of LTBI to prevent its progression to active disease. Traditionally, prevention of tuberculosis has relied on public health systems; however, more recently, screening for LTBI has become a relevant primary care issue.
DETECTION
The USPSTF found adequate evidence that accurate screening tests are available to detect LTBI. Screening tests include the Mantoux tuberculin skin test (TST) and interferon-gamma release assays (IGRAs); both are moderately sensitive and highly specific.
BENEFITS OF EARLY DETECTION AND TREATMENT
The USPSTF found no studies that evaluated the direct benefits of screening for LTBI. The USPSTF found adequate evidence that treatment of LTBI with regimens recommended by the Centers for Disease Control and Prevention (CDC) decreases progression to active tuberculosis; the magnitude of this benefit is moderate.
HARMS OF EARLY DETECTION AND TREATMENT
The USPSTF found no direct evidence on the harms of screening for LTBI. The USPSTF found adequate evidence that the magnitude of harms of treatment of LTBI with CDC-recommended regimens is small. The primary harm of treatment is hepatotoxicity.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that the net benefit of screening for LTBI in persons at increased risk for tuberculosis is moderate.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to asymptomatic adults 18 years and older at increased risk for tuberculosis (see the “Assessment of Risk” section for more information). It does not apply to adults with symptoms of tuberculosis or to children and adolescents.
ASSESSMENT OF RISK
Populations at increased risk for LTBI based on increased prevalence of active disease and increased risk of exposure include persons who were born in, or are former residents of, countries with increased tuberculosis prevalence and persons who live in, or have lived in, high-risk congregate settings (e.g., homeless shelters and correctional facilities). Clinicians can consult their local or state health departments for more information about populations at risk in their community, because local demographic patterns may vary across the United States.
In 2015, among persons of known national origin, 66.2% of all active tuberculosis cases in the United States were among foreign-born persons, and the case rate of active tuberculosis among foreign-born persons was approximately 13 times higher than among U.S.-born persons (15.1 vs. 1.2 cases per 100,000 persons).7 More than half of all foreign-born persons in the United States with active tuberculosis were from 5 countries: Mexico, the Philippines, Vietnam, India, and China.7 In addition, the CDC has identified foreign-born persons from Haiti and Guatemala as important contributors to active tuberculosis cases in the United States.8 The World Health Organization recently updated its list of countries with a high burden of tuberculosis to include the top 20 countries with the highest absolute numbers of cases and an additional 10 countries with the most severe burden in terms of case rate per capita.9
Persons who live in, or have lived in, high-risk congregate settings also have a higher prevalence rate of active tuberculosis and increased risk for exposure. Among persons 15 years and older with active tuberculosis, 5.6% were homeless within the past year, 2.2% were residents of a long-term care facility, and 4.2% were in a correctional facility at the time of diagnosis.10 Published prevalence rates of LTBI in these settings vary widely, depending on the type of screening test used, the TST threshold used to define the presence of LTBI, and the population studied. Estimates of LTBI prevalence range from 23.1% to 87.6% among prisoners and from 18.6% to 79.8% among persons who are homeless.2,11
Other populations at increased risk for LTBI or progression to active disease include persons who are immunosuppressed (e.g., persons living with human immunodeficiency virus [HIV], patients receiving immunosuppressive medications such as chemotherapy or tumor necrosis factor-alpha inhibitors, and patients who have received an organ transplant) and patients with silicosis (a lung disease). However, given that screening in these populations may be considered standard care as part of disease management or indicated prior to the use of certain medications, the USPSTF did not review evidence on screening in these populations. Some evidence from observational studies has explored the association between poorly controlled diabetes and progression of LTBI to active disease. However, there is insufficient evidence on screening for and treatment of LTBI in persons with diabetes for the USPSTF to make a separate recommendation for this important subgroup.
Persons who are contacts of individuals with active tuberculosis, health care workers, and workers in high-risk congregate settings may also be at increased risk of exposure. Since screening in these populations is conducted as part of public health12 or employee health13,14 surveillance, the USPSTF did not review the evidence in these populations. Clinicians seeking further information about testing for tuberculosis in these populations can refer to the “Useful Resources” and “Recommendations of Others” sections.
SCREENING TESTS
Two types of screening tests for LTBI are currently available in the United States: the TST and IGRA. The TST requires intradermal placement of purified protein derivative and interpretation of response 48 to 72 hours later. The skin test reaction is measured in millimeters of the induration (a palpable, raised, hardened area or swelling). Interferon-gamma release assays require a single venous blood sample and laboratory processing within 8 to 30 hours after collection. Two types of IGRAs are currently approved by the U.S. Food and Drug Administration: T-SPOT.TB (Oxford Immunotec Global) and QuantiFERON-TB Gold In-Tube (Qiagen).
Numerous patient and systems factors may influence the selection of a screening test.15 Generally, the CDC recommends screening with either the TST or IGRA but not both. Testing with IGRAs may be preferable for persons who have received a bacille Calmette-Guérin vaccination or persons who may be unlikely to return for TST interpretation. Additional information on the use and interpretation of the TST and IGRA is available from the CDC.16
SCREENING INTERVALS
The USPSTF found no evidence on the optimal frequency of screening for LTBI. Depending on specific risk factors, screening frequency could range from 1-time only screening among persons who are at low risk for future tuberculosis exposure to annual screening among those who are at continued risk of exposure.
TREATMENT
Recommendations for the treatment of LTBI are available from the CDC.17
ADDITIONAL APPROACHES TO PREVENTION
The public health system has an essential role in the control and elimination of tuberculosis. Clinicians are required to report cases of active tuberculosis to their local health department. As outlined by local and state public health laws, local health departments investigate and ensure treatment of active tuberculosis cases and perform contact tracing and medical surveillance of contacts.
Occupational health services also have an important role in the prevention and control of tuberculosis. Certain work settings (health care settings, correctional facilities, and other high-risk congregate housing settings) may pose a higher risk of exposure, and employers often have an important role in preventing tuberculosis exposure among employees and performing medical surveillance of employees for exposure.
USEFUL RESOURCES
Clinicians seeking guidance on tuberculosis management among persons living with HIV can obtain additional information from the National Institutes of Health.18 Clinicians seeking information on medical surveillance of contacts of persons with active tuberculosis can contact their local health department, review their local public health law, or review guidance from the CDC.19 The CDC also provides information for public health tuberculosis programs.20
Clinicians seeking information on medical surveillance of health care workers or employees working in high-risk settings can consult resources from the CDC and the Occupational Safety and Health Administration.21–23 Clinicians seeking guidance on screening for LTBI in children can find more information on the American Academy of Pediatrics' Bright Futures website.24 Clinicians seeking guidance on tuberculosis and pregnancy can obtain information from the CDC.25
This recommendation statement was first published in JAMA. 2016; 316(9):962–969.
The “Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
