
Am Fam Physician. 2017;95(8):507-513
NOTE: Since the publication of this article, the Centers for Disease Control and Prevention (CDC) has released updated interim guidelines for pregnant women with possible Zika virus exposure and diagnosis, evaluation. and mangement of infants with possible congential Zika virus infection. Current information on Zika virus for clincians is available on the CDC's web site.
Author disclosure: No relevant financial affiliations.
Since local mosquito-borne transmission of Zika virus was first reported in Brazil in early 2015, the virus has spread rapidly, with active transmission reported in at least 61 countries and territories worldwide, including the United States. Zika virus infection during pregnancy is a cause of microcephaly and other severe brain anomalies. The virus is transmitted primarily through the bite of an infected Aedes mosquito, but other routes of transmission include sexual, mother-to-fetus during pregnancy, mother-to-infant at delivery, laboratory exposure, and, possibly, transfusion of blood products. Most persons with Zika virus infection are asymptomatic or have only mild symptoms; hospitalizations and deaths are rare. When symptoms are present, maculopapular rash, fever, arthralgia, and conjunctivitis are most common. Zika virus testing is recommended for persons with possible exposure (those who have traveled to or live in an area with active transmission, or persons who had sex without a condom with a person with possible exposure) if they have symptoms consistent with Zika virus disease. Testing is also recommended for pregnant women with possible exposure, regardless of whether symptoms are present. Treatment is supportive, and no vaccine is currently available. The primary methods of prevention include avoiding bites of infected Aedes mosquitoes and reducing the risk of sexual transmission. Pregnant women should not travel to areas with active Zika virus transmission, and men and women who are planning to conceive in the near future should consider avoiding nonessential travel to these areas. Condoms can reduce the risk of sexual transmission.
Zika virus is a single-stranded RNA flavivirus closely related to dengue, yellow fever, and West Nile viruses, and it is most commonly transmitted through the bite of infected Aedes (Stegomyia) mosquitoes.1 Zika virus was first isolated in Uganda in 1947, but has only been recognized to cause large outbreaks of human disease since 2007.2,3 Several months after a 2015 outbreak of Zika virus in Brazil,4 reports of microcephaly in newborns increased.5 A subsequent evidence review using a systematic framework concluded that prenatal Zika virus infection causes microcephaly and other serious brain anomalies.6 Further epidemiologic and experimental evidence that Zika virus causes birth defects has accumulated.7–9
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| For pregnant women (symptomatic* and asymptomatic) who are evaluated two to 12 weeks after possible exposure to Zika virus,† serum IgM testing is recommended. If results are positive or equivocal, serum and urine RNA NAT should be performed. If RNA NAT results are negative, PRNT should be performed. | C | 15 |
| Asymptomatic pregnant women who live in areas with active Zika virus transmission should have Zika virus IgM testing as part of routine obstetric care during the first and second trimesters, with immediate RNA NAT for those with positive IgM results. | C | 15 |
| For symptomatic* persons (including pregnant women) with possible exposure to Zika virus† who present less than two weeks after symptom onset, serum and urine RNA NAT is recommended. If results are negative, serum IgM testing should be performed, and a positive or equivocal IgM result should be confirmed by PRNT. | C | 39 |
| For asymptomatic pregnant women with possible exposure to Zika virus† who do not live in areas with active Zika virus transmission, serum and urine RNA NAT is recommended if the samples are collected less than two weeks after the last possible exposure. If results are negative, Zika virus IgM testing should be performed on serum collected two to 12 weeks after possible exposure, and a positive or equivocal IgM result should be confirmed by PRNT. | C | 15 |
| For symptomatic* persons with possible exposure to Zika virus† who present two weeks or more after symptom onset, serum IgM testing should be performed. In nonpregnant women, a positive or equivocal IgM result should be followed by PRNT to confirm the diagnosis. | C | 15 |
| Pregnant women should not travel to areas with active Zika virus transmission. | C | 15 |
| Men and women who do not live in areas with active Zika virus transmission and who are planning to conceive in the near future should consider avoiding nonessential travel to areas with active Zika virus transmission. | C | 25 |
| All persons who live in or travel to an area with active Zika virus transmission should be counseled on strategies to prevent transmission of Zika virus and other mosquito-borne diseases. This includes using Environmental Protection Agency–registered insect repellents, wearing long-sleeved shirts and long pants, using permethrin-treated clothing (except in Puerto Rico), and using air conditioning or window and door screens when indoors. | C | 1 |
| Pregnant women with partners who live in or have traveled to an area with active Zika virus transmission should use condoms every time they have sex or should abstain from sex with that partner for the remainder of the pregnancy. | C | 25 |
| Women of reproductive age who have had or anticipate Zika virus exposure and do not want to become pregnant should be counseled about contraceptive options. | C | 25, 46 |
| Men who do not live in an area with active Zika virus transmission but have possible Zika virus exposure† should wait to attempt conception with their partner for at least six months after symptom onset or the last possible exposure. | C | 25 |
| Women who do not live in areas with active Zika virus transmission but have possible Zika virus exposure† should wait to attempt conception until at least eight weeks after symptom onset or last possible exposure. | C | 25 |
| Women who live in an area with active Zika virus transmission and who have symptoms should be tested for Zika virus infection; those who test positive should wait at least eight weeks from symptom onset to attempt conception. | C | 25 |
| Men who live in an area with active Zika virus transmission and who have symptoms should be tested for Zika virus infection; those who test positive should wait at least six months from symptom onset to attempt conception. | C | 25 |
| Asymptomatic women and men who live in areas with active Zika virus transmission and who desire pregnancy should talk with their physician. | C | 25 |
A recognizable pattern of anomalies has emerged: congenital Zika syndrome includes severe microcephaly with misshapen skull, scalp rugae, intracranial calcifications and other brain anomalies, eye anomalies (e.g., chorioretinal atrophy, scarring), clubfoot, joint contractures (arthrogryposis), and neurologic sequelae (e.g., vision loss, hearing impairment, motor and cognitive disabilities, seizures, swallowing difficulties, hypertonia, spasticity with tremors, irritability).10 It is not known whether congenitally infected infants without congenital Zika syndrome at birth will develop problems later in life. Interim guidance for evaluation and management of infants born to mothers with Zika virus infection during pregnancy is available.11
Congenital Zika syndrome is associated with infection in the first and early second trimesters of pregnancy, but later infection may also be associated with adverse outcomes. The risk of microcephaly in infants born to women with prenatal Zika virus infection is estimated at 1% to 13%.12–14 Modifying risk factors have not been identified. Information about pregnant women with laboratory evidence of Zika virus infection should be collected for inclusion in the U.S. Zika Pregnancy Registry (for all U.S. states and territories except Puerto Rico) or the Zika Active Pregnancy Surveillance System (for Puerto Rico). These registries were established to update clinical recommendations, plan for services for pregnant women and their families, and improve prevention of Zika virus infection during pregnancy.15
Other complications associated with Zika virus infection include Guillain-Barré syndrome,16 an autoimmune form of flaccid paralysis, and thrombocytopenia.17 An association between Zika virus infection and Guillain-Barré syndrome was first noted during an outbreak in French Polynesia in 2013–2014.16 Data from other countries have supported this association,18,19 and an expert panel convened by the World Health Organization recently concluded that Zika virus infection is a trigger for Guillain-Barré syndrome.9 The risk after Zika virus infection is estimated at 0.24 cases per 1,000.16 The median time from Zika-related symptom onset to the onset of neurologic symptoms is five to six days.16,18 Other neurologic manifestations such as encephalitis have been reported after Zika virus infection,16,18 but their association is less well understood. Acute Zika virus illness is rarely associated with severe, possibly life-threatening thrombocytopenia.17 Clinically distinguishing between Zika virus and dengue virus infections may be particularly difficult in patients with thrombocytopenia and hemorrhage.
Since mosquito-borne transmission of Zika virus was first reported in Brazil in early 2015, the virus has spread rapidly; as of March 3, 2017, at least 61 countries and territories worldwide had active mosquito-borne transmission, including the United States.20 As of March 3, 2017, travel-associated cases had been reported in all states except Alaska, and clusters of mosquito-borne transmission have been identified in areas of Florida and Texas. Based on the distribution of Aedes mosquitoes,1 mosquito-borne transmission is possible in other areas of the United States. Clinicians should be familiar with Zika virus, its associated complications, and prevention strategies. This article addresses common questions about Zika virus and provides evidence-based answers. Updated information on Zika virus is available at http://www.cdc.gov/zika.
What Are the Signs and Symptoms of Zika Virus Infection?
Most persons with Zika virus infection are asymptomatic. Among those reporting symptoms, rash, fever, arthralgia, and conjunctivitis are most common. Infection is self-limited, rarely extending beyond one week.3
EVIDENCE SUMMARY
Based on data from a Zika virus outbreak in 2007, only 18% of infected persons had a clinical illness that was thought to be related to Zika virus.3 Among those with symptoms, maculopapular rash was most common, followed by fever, arthritis or arthralgia, and nonpurulent conjunctivitis.3,21 Other reported findings include myalgia, headache, retro-orbital pain, edema, and vomiting. The median incubation period is approximately six days,22 and symptoms typically last from several days to one week. Zika virus–related hospitalizations and deaths are rare.1 Manifestations in children are similar to those in adults.23
Who Should Be Tested for Zika Virus?
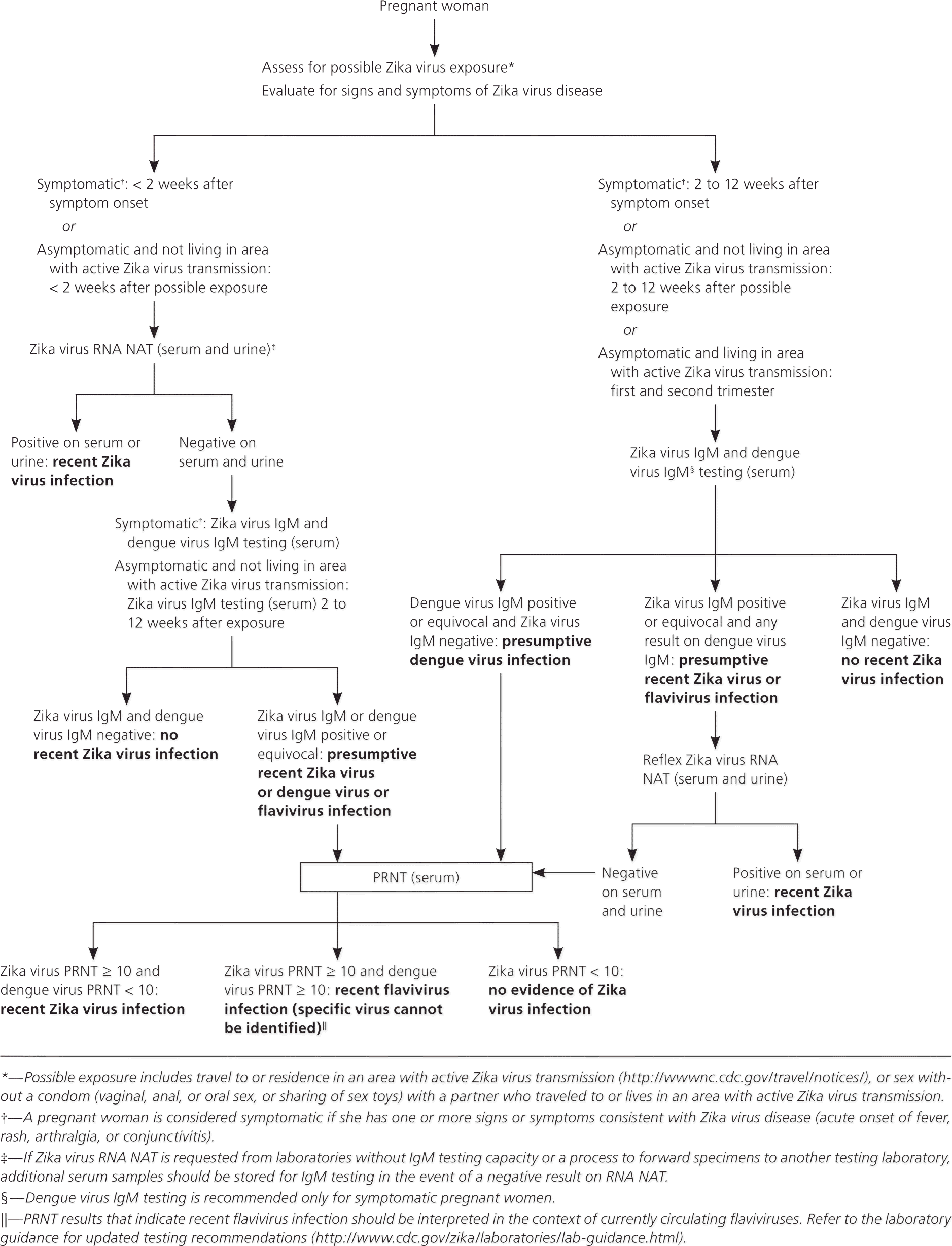
Testing is recommended for persons with possible exposure to Zika virus (defined as travel to or residence in an area with active transmission or sex without a condom with a partner who has traveled to or lived in an area with active transmission) if they have experienced or are experiencing symptoms consistent with Zika virus infection, or if they are pregnant, regardless of the presence of symptoms (Figure 1). 24 All pregnant women should be asked at each prenatal care visit about possible exposure to Zika virus.
EVIDENCE SUMMARY
The primary mode of Zika virus transmission is through the bite of infected Aedes aegypti (more common) or Aedes albopictus mosquitoes. Sexual transmission has also been documented, including transmission from both men and women and from symptomatic and asymptomatic persons.25 It is not known how long semen can remain infectious after Zika virus infection. Suspected sexual transmission has occurred 32 to 41 days after symptom onset. Zika virus has been cultured in semen (which may indicate infectiousness) up to 69 days after symptom onset, and low levels of Zika virus RNA have been detected up to 188 days after symptom onset (although the relationship between RNA detection and infectivity is not known).25
In addition to mother-to-fetus transmission of Zika virus during pregnancy,26,27 transmission at delivery has been documented in two infants; one was asymptomatic, and the other had mild illness.28 Transmission through laboratory exposures has been documented.29 Probable transmission through transfusion of platelets has been reported in Brazil.30,31 All blood donations in the United States and U.S. territories are currently screened for Zika virus to decrease transfusion-associated risk.32
Zika virus has been identified in saliva, urine, and breast milk, but transmission through these sources has not been reported.33–35 Infection was reported in a person from Utah with no travel or sexual exposure history; no transmission mode was identified, but transmission may have occurred through close contact with a person with an unusually high viral load.36,37 Transmission to health care professionals has not been reported. Persons working in health care and laboratory settings should use standard precautions to prevent infection.38
What Tests Are Available for Detecting Zika Virus Infection?
Testing to determine possible Zika virus infection should be limited to specimens collected from patients who meet clinical and epidemiologic criteria from the Centers for Disease Control and Prevention.39 Testing is complicated by the temporal appearance and disappearance of viral RNA and development of virus-specific immunoglobulin M (IgM) and neutralizing antibodies, and by serologic cross-reactivity between Zika virus and related flaviviruses. Depending on when specimens are collected, multiple tests and sample types may be needed for a definitive laboratory diagnosis. 40 The Centers for Disease Control and Prevention has published interim guidelines for testing at-risk populations, including information about appropriate samples, timing of sample collection, and tests to be performed.41
EVIDENCE SUMMARY
For most persons with Zika virus disease, RNA is expected to be present in the serum from several days before symptom onset until one week afterward. The timing after infection and duration of RNA detectability are expected to be similar in asymptomatic infected persons, but substantiating data are lacking.40 In some pregnant women, Zika virus RNA has been detected for longer periods—up to 10 weeks after symptom onset in one case.27,42 Limited data suggest that Zika virus RNA may be detected in urine up to two weeks after symptom onset.43 Thus, blood and urine of symptomatic persons—including pregnant women—with possible Zika virus exposure, and of asymptomatic pregnant women with possible exposure who do not live in areas with active transmission, should be obtained for RNA nucleic acid testing less than two weeks after symptom onset or last exposure.15 A positive test confirms infection, but a negative test does not exclude infection and should be followed by serologic testing. If samples are collected two weeks or more after symptom onset, serologic testing should be performed; pregnant women with positive or equivocal Zika virus IgM antibodies should undergo immediate RNA nucleic acid testing.15 Although serum and urine are the primary specimens for Zika virus testing, other specimens (e.g., plasma, whole blood, cerebrospinal fluid, amniotic fluid) are approved for some tests.
Zika virus IgM antibodies typically appear in the first week of illness, but it is not known how long they persist. Experience with other flaviviruses suggests that IgM remains detectable for at least three months after infection.1 Zika virus–neutralizing antibodies, which consist primarily of immunoglobulin G, appear shortly after IgM antibodies and are expected to remain for years and possibly for life.40 Zika virus antibody testing is challenging to interpret because of cross-reactivity with other flaviviruses, especially among persons previously infected with or vaccinated against a related flavivirus. Confirmatory plaque reduction neutralization testing is recommended for persons with positive or equivocal Zika virus IgM antibodies; however, this test is not routinely recommended in Puerto Rico because dengue virus is endemic, and cross-reactivity will occur in most cases.40
How Is Zika Virus Disease Treated?
No specific treatment is available for Zika virus disease. 1
EVIDENCE SUMMARY
Treatment of Zika virus disease is supportive and includes rest, hydration, and use of antipyretics and analgesics. Because of the risk of hemorrhage, aspirin and other nonsteroidal anti-inflammatory drugs should be avoided until dengue virus infection is excluded (http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf?ua=1).
How Can Zika Virus Infection Be Prevented?
There is no approved vaccine for Zika virus. The primary methods of prevention are avoiding the bites of infected Aedes mosquitoes and reducing the risk of sexual transmission. Pregnant women should not travel to areas with active Zika virus transmission and should abstain from sex or use condoms for the duration of pregnancy if their partner has possible Zika virus exposure.15 Men and women who do not live in areas with active Zika virus transmission and who are planning to conceive in the near future should consider avoiding nonessential travel to areas with active transmission.25 If they have possible exposure, they should wait at least six months (for men) or at least eight weeks (for women) from symptom onset or last possible exposure before attempting conception.
EVIDENCE SUMMARY
Although no vaccine for Zika virus has been approved, several approaches to vaccine development are being pursued.44 Mosquito bites can be reduced by wearing long-sleeved shirts and long pants, using Environmental Protection Agency–registered mosquito repellents (https://www.epa.gov/insect-repellents/find-insect-repellent-right-you), using permethrin-treated clothing (except in Puerto Rico because of resistance), and using air conditioning or screens on doors and windows.1 Individual and community efforts to decrease mosquito populations are also needed.45 Persons with Zika virus infection should be counseled to avoid mosquito bites during the first few weeks of illness to reduce the risk of human-to-mosquito-to-human transmission; counseling should also address the use of condoms or abstinence for at least eight weeks for women and at least six months for men after illness onset or last possible exposure to prevent sexual transmission.25
Sex without a condom with partners at risk of Zika virus infection should be avoided. Pregnant women and their partners who live in or traveled to an area with active Zika virus transmission should use condoms every time they have sex or abstain from sex for the remainder of the pregnancy.25 Women of reproductive age who anticipate exposure to Zika virus and who do not desire pregnancy should be counseled about contraceptive options to prevent unintended pregnancy; selection should be based on safety, effectiveness, availability, and acceptability.46
For couples with Zika virus disease or possible exposure who do not live in an area with active Zika virus transmission and who are planning a pregnancy, a waiting period is recommended. Men should not attempt conception for at least six months after symptom onset or last possible exposure, and women should delay conception at least eight weeks after symptom onset or last possible exposure.25 Women and men who live in areas with active Zika virus transmission and have Zika virus disease should wait at least eight weeks and six months, respectively, after symptom onset before attempting to conceive.25 Those who live in areas with active transmission, do not have Zika virus disease, and desire pregnancy should talk with their physician.25 Guidance for clinicians is available at https://www.cdc.gov/zika/pdfs/preconception-counseling.pdf, and Table 1 lists resources for patients.
| American Academy of Pediatrics |
| https://www.healthychildren.org/English/ages-stages/prenatal/Pages/Zika-Virus.aspx |
| American College of Obstetricians and Gynecologists |
| http://www.acog.org/About-ACOG/ACOG-Departments/Zika-Virus/Resources-for-Patients |
| Centers for Disease Control and Prevention |
| http://www.cdc.gov/zika |
| Familydoctor.org |
| https://familydoctor.org/condition/zika/ |
| March of Dimes |
| http://www.marchofdimes.org/complications/zika-virus-and-pregnancy.aspx?utm_source=homeslide&utm_medium=MOD-site&utm_campaign=MOD&utm_content=Zika-article |
| MotherToBaby |
| https://mothertobaby.org/fact-sheets/zika-virus-pregnancy/pdf |
| U.S. Environmental Protection Agency |
| https://www.epa.gov/mosquitocontrol |
| World Health Organization |
| http://www.who.int/csr/disease/zika/en |
Data Sources: Primary sources included the Centers for Disease Control and Prevention website (http://www.cdc.gov/zika) and interim guidance documents published in Morbidity and Mortality Weekly Report (http://www.cdc.gov/mmwr). This information was supplemented by articles identified through a PubMed search using the key term Zika virus and the following additional terms: pregnancy, microcephaly, Guillain-Barré syndrome, epidemiology, surveillance, transmission, diagnostics, and prevention. The results of this search consisted of case reports and series, cohort studies, case-control studies, and narrative and systematic review articles. Search dates: June 17, 2016, to January 15, 2017.
