
Am Fam Physician. 2017;95(8):521-522
Author disclosure: No relevant financial affiliations.
Albiglutide (Tanzeum) is a glucagon-like peptide 1 (GLP-1) receptor agonist labeled as an adjunct to diet and exercise for the management of type 2 diabetes mellitus in adults. As with other GLP-1 receptor agonists, albiglutide stimulates postmeal insulin secretion and slows gastric emptying to promote satiety.1 Albiglutide can be used alone or in combination with metformin, sulfonylureas, thiazolidinediones, or basal insulin.2–5 It has not been studied in combination with prandial insulin.
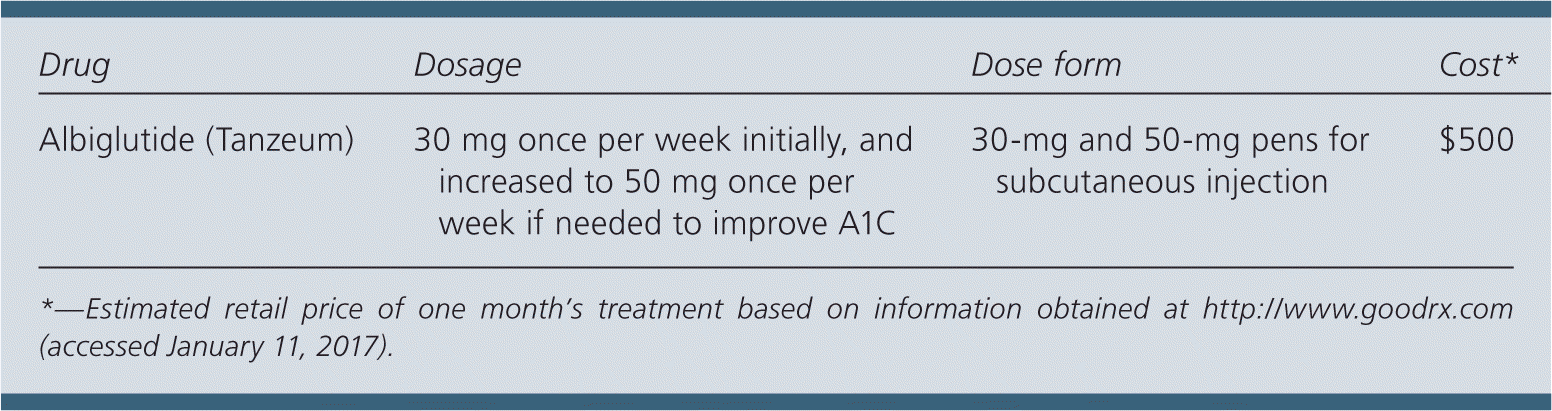
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Albiglutide (Tanzeum) | 30 mg once per week initially, and increased to 50 mg once per week if needed to improve A1C | 30-mg and 50-mg pens for subcutaneous injection | $500 |
SAFETY
As with other GLP-1 receptor agonists, use of albiglutide increases the risk of acute pancreatitis (0.3% with albiglutide vs. 0.1% with placebo).1 Although the risk of hypoglycemia is low (2% over one year) with albiglutide monotherapy, the risk increases when it is added to a sulfonylurea (13% over one year) or basal insulin (16% over six months).1 No dosage adjustment is needed in patients with renal dysfunction, although albiglutide should not be used in patients with end-stage renal disease.6 Renal failure has been reported in dehydrated patients. As with several other GLP-1 receptor agonists, albiglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma, and in patients with multiple endocrine neoplasia syndrome type 2.1 Albiglutide is a U.S. Food and Drug Administration pregnancy category C drug and has not been evaluated in breastfeeding women.1
TOLERABILITY
Overall, albiglutide is well tolerated, with adverse effect rates similar to placebo. Gastrointestinal symptoms, including nausea, vomiting, diarrhea, constipation, and acid reflux, are the most common adverse effects (39% with albiglutide vs. 33% with placebo) and are most often mild.1 Injection site reactions are also more common with albiglutide than with placebo (18% vs. 8%).1 In clinical trials, 2% of patients discontinued albiglutide because of adverse effects.1
EFFECTIVENESS
Albiglutide has not been studied to determine its effects on mortality or on macro-vascular manifestations of type 2 diabetes. Used alone, albiglutide reduces A1C by 0.5 to 0.8 percentage points and fasting blood glucose by about 16 mg per dL (0.89 mmol per L).1–5 This is similar to expected reductions with glimepiride (Amaryl); sitagliptin (Januvia); pioglitazone (Actos); and liraglutide (Victoza), another GLP-1 agonist.2–5,7 In patients with renal impairment, albiglutide has superior A1C reduction compared with sitagliptin.8 Albiglutide retains its effectiveness when used with basal insulin or oral therapies, with A1C reduction similar to monotherapy.2–5 Patients taking albiglutide will have an average weight loss of 0.4 to 1.1 kg (14 oz to 2 lb, 3 oz) by one year.4,5
PRICE
A 30-day supply of albiglutide (four 30-mg or 50-mg injections) will cost about $500, which is similar to other GLP-1 receptor agonists. None of the five medications in this class is available generically, and a patient's third-party formulary should be investigated before prescribing.
SIMPLICITY
Albiglutide is administered by subcutaneous injection, once weekly with or without food, beginning with the 30-mg dose and increasing to 50 mg if needed to improve A1C. Patients will need to prepare the product 15 to 30 minutes before injection using supplied materials and instructions.
Bottom Line
Reductions in A1C are less with albiglutide than with the first-line therapy metformin, and albiglutide costs significantly more than metformin and sulfonylureas. Albiglutide's ability to reduce diabetes-related morbidity and major cardiovascular events is unknown. Like other GLP-1 agonists, albiglutide may provide some A1C benefit as an adjunct with a low risk of adverse effects. Albiglutide's once-weekly dosing may be useful for patients with compliance challenges. Until more is known regarding long-term patient-oriented outcomes, albiglutide should be used only as a second- or third-line agent in patients who are comfortable giving themselves an injection and desire once-weekly treatment.
