
Am Fam Physician. 2017;96(1):22A-22B
Author disclosure: No relevant financial affiliations.

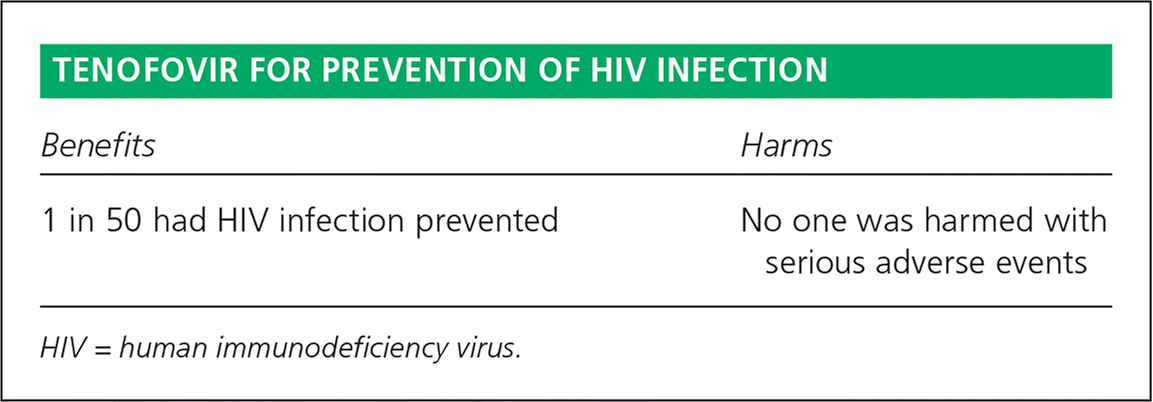
| Benefits | Harms |
|---|---|
| 1 in 50 had HIV infection prevented | No one was harmed with serious adverse events |
Details for This Review
Study Population: Human immunodeficiency virus (HIV)-negative commercial sex workers, men who have sex with men, and individuals with an HIV-positive partner, mostly in developing settings
Efficacy End Points: Incidence of HIV infection; adherence to preexposure prophylaxis (PrEP)
Harm End Points: Adverse events including renal insufficiency, elevated liver enzymes, nausea, dizziness, and vomiting
Narrative: HIV infection occurs in 2.7 million persons each year globally. There remains a need for effective prevention strategies. In 2012, the U.S. Food and Drug Administration approved tenofovir/emtricitabine (Truvada) in the United States for PrEP in conjunction with safer sex practices to reduce the risk of HIV transmission. Nonetheless, research into PrEP has stirred controversy because of concerns about perpetuating risky sexual behaviors, long-term medication effects, and creating drug-resistant strains of HIV from poor compliance.
This Cochrane review reports on the results of six trials examining prevention of HIV infection in persons at high risk.1 This review concluded that compared with placebo, tenofovir/emtricitabine (n = 8,918; relative risk [RR] = 0.5; 95% confidence interval [CI], 0.3 to 0.9) and tenofovir alone (n = 4,027; RR = 0.33; 95% CI, 0.2 to 0.6) both significantly reduced the risk of HIV infection. Moreover, serious adverse events were similar for patients in the PrEP and placebo groups, although minor adverse events such as nausea and vomiting were significantly more common in those receiving medications. The Cochrane review does not address the rate of minor adverse events. However, the original studies report rates between 1% and 10% for minor adverse events such as headache, nausea, vomiting, or dizziness.2–4
The number needed to treat (NNT) is relatively high because even in these very high-risk groups the annual infection rate was just 4%, perhaps because of safe sex and other prevention education in all groups. The infection rate was roughly 2% in the medication groups, for a raw difference of 2% and an NNT of 50.
Caveats: The studies included here were performed largely in developing countries, where the global new infection burden is highest. It is not yet clear how well this will translate in other settings. Median follow-up varied, typically one to two years; therefore, long-term impact and effectiveness are also unknown. These issues may be better clarified by results from ongoing studies.
Tenofovir/emtricitabine is associated with kidney toxicity (reversed when the drug was discontinued in these studies), loss of bone density, and liver inflammation in patients with hepatitis B. There is concern that using tenofovir before transmission of HIV may limit its effectiveness in treating infection later. If persons believe the medication obviates the need for safe behaviors, the intervention could paradoxically increase HIV transmission. A final consideration is cost. As of November 2016, the cost of tenofovir/emtricitabine is approximately $1,000 for a 30-day supply (30 tablets) in the United States.
We have labeled the intervention green (benefits greater than harm), but cost-effectiveness and personal preferences are critical. Treating this target population with HIV drugs so that 2% can avoid infection (and thus avoid the same drugs) may not be a worthy trade-off for some, particularly if adverse events are vexing. For others, the peace of mind offered by taking the medication may be more than worthwhile. The duration of prophylaxis is indefinite or as long as the high-risk exposure continues.