
Am Fam Physician. 2017;96(2):online
Related Putting Prevention into Practice: Screening for Obstructive Sleep Apnea in Adults.
As published by the U.S. Preventive Services Task Force.
Summary of Recommendation and Evidence
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for obstructive sleep apnea (OSA) in asymptomatic adults (Table 1). I statement.
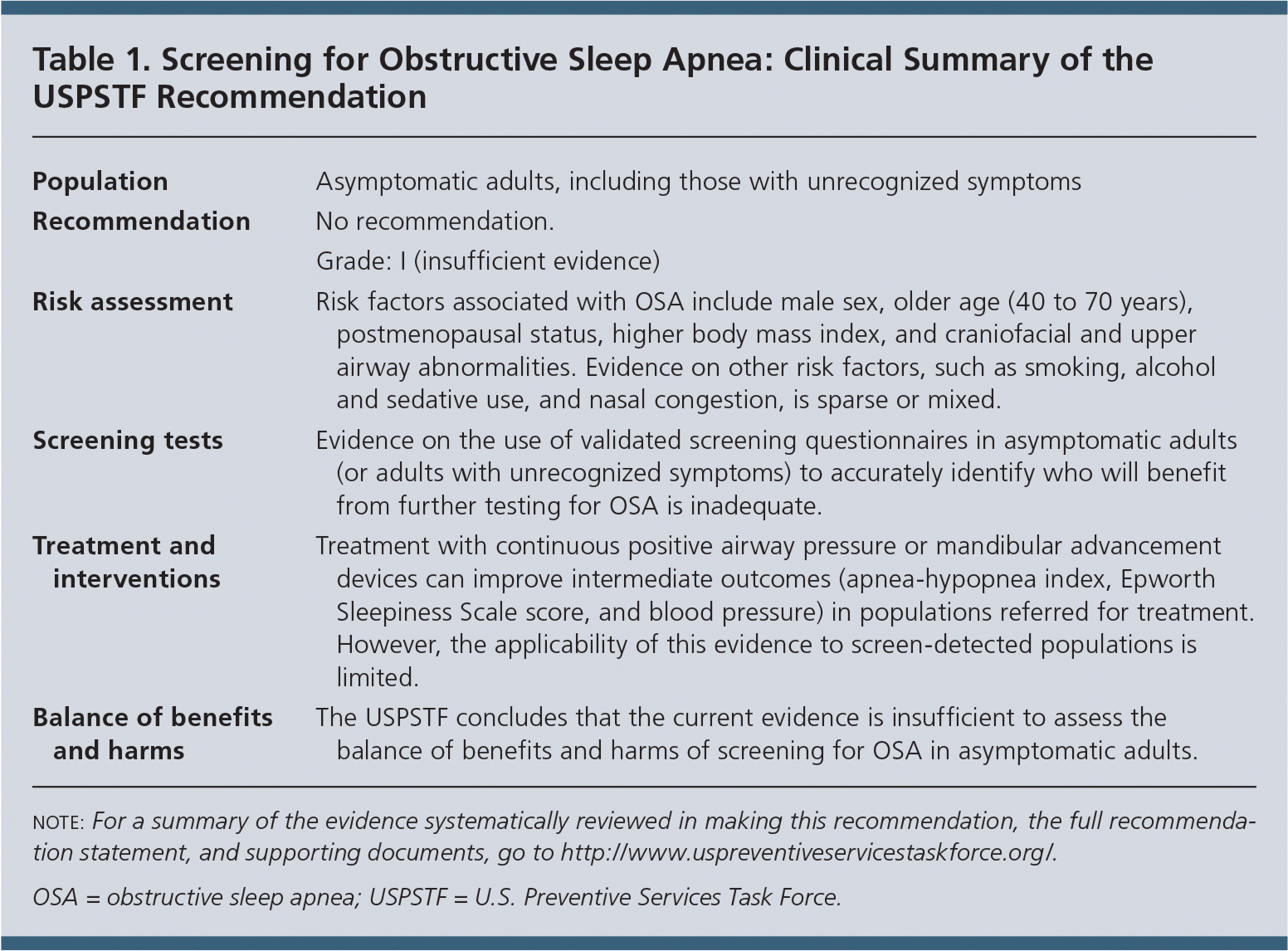
| Population | Asymptomatic adults, including those with unrecognized symptoms |
| Recommendation | No recommendation. |
| Grade: I (insufficient evidence) | |
| Risk assessment | Risk factors associated with OSA include male sex, older age (40 to 70 years), postmenopausal status, higher body mass index, and craniofacial and upper airway abnormalities. Evidence on other risk factors, such as smoking, alcohol and sedative use, and nasal congestion, is sparse or mixed. |
| Screening tests | Evidence on the use of validated screening questionnaires in asymptomatic adults (or adults with unrecognized symptoms) to accurately identify who will benefit from further testing for OSA is inadequate. |
| Treatment and interventions | Treatment with continuous positive airway pressure or mandibular advancement devices can improve intermediate outcomes (apnea-hypopnea index, Epworth Sleepiness Scale score, and blood pressure) in populations referred for treatment. However, the applicability of this evidence to screen-detected populations is limited. |
| Balance of benefits and harms | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for OSA in asymptomatic adults. |
Go to the Clinical Considerations section for suggestions for practice regarding the I statement.
Rationale
IMPORTANCE
Based on data from the 1990s, the estimated prevalence of OSA in the United States is 10% for mild OSA and 3.8% to 6.5% for moderate to severe OSA.1–3 Current prevalence may be higher, given the increasing prevalence of obesity.4,5 The proportion of persons with OSA who are asymptomatic or have unrecognized symptoms is unknown. Severe OSA is associated with increased all-cause mortality 6; however, the role OSA plays in increasing overall mortality, independent from other risk factors (older age, higher body mass index [BMI], and other cardiovascular risk factors), is less clear. In addition to mortality, other adverse health outcomes associated with untreated OSA include cardiovascular disease and cerebrovascular events, diabetes, cognitive impairment, decreased quality of life, and motor vehicle crashes.
DETECTION
Evidence on the use of validated screening questionnaires in asymptomatic adults (or adults with unrecognized symptoms) to accurately identify who will benefit from further testing for OSA is inadequate. The USPSTF identified this as a critical gap in the evidence.
BENEFITS OF EARLY DETECTION AND INTERVENTION OR TREATMENT
The USPSTF found inadequate direct evidence on the benefit of screening for OSA in asymptomatic populations. The USPSTF found no studies that evaluated the effect of screening for OSA on health outcomes. The USPSTF found at least adequate evidence that treatment with continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) can improve intermediate outcomes (e.g., the apnea-hypopnea index [AHI], Epworth Sleepiness Scale [ESS] score, and blood pressure) in populations referred for treatment. However, the applicability of this evidence to screen-detected populations is limited. The adequacy of the evidence varies based on the type of intervention and the reported intermediate outcomes. The USPSTF found inadequate evidence on the link between change in the intermediate outcome (e.g., AHI) and reduction in the health outcome (e.g., mortality). The USPSTF found evidence that treatment with CPAP can improve general and sleep-related quality of life in populations referred for treatment, but the applicability of this evidence to screen-detected populations is unknown. The USPSTF found inadequate evidence on whether treatment with CPAP or MADs improves other health outcomes (mortality, cognitive impairment, motor vehicle crashes, and cardiovascular or cerebrovascular events). The USPSTF also found inadequate evidence on the effect of treatment with various surgical procedures in improving intermediate or health outcomes.
HARMS OF EARLY DETECTION AND INTERVENTION OR TREATMENT
The USPSTF found inadequate evidence on the direct harms of screening for OSA. The USPSTF found adequate evidence that the harms of treatment of OSA with CPAP and MADs are small. Reported harms include oral or nasal dryness; eye or skin irritation; rash; epistaxis; pain; excess salivation; and oral mucosal, dental, and jaw symptoms. The USPSTF found inadequate evidence on the harms of surgical treatment of OSA.
USPSTF ASSESSMENT
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for OSA in asymptomatic adults. Evidence on screening tools to accurately detect persons in asymptomatic populations who should receive further testing and treatment of subsequently diagnosed OSA to improve health outcomes is lacking, and the balance of benefits and harms cannot be determined.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to asymptomatic adults (18 years and older). It also applies to adults with unrecognized symptoms of OSA. This includes persons who are not aware of their symptoms or do not report symptoms as being a concern to their clinician. This recommendation does not apply to persons presenting with symptoms (e.g., snoring, witnessed apnea, excessive daytime sleepiness, impaired cognition, mood changes, or gasping or choking at night) or concerns about OSA, persons who have been referred for evaluation or treatment of suspected OSA, or persons who have acute conditions that could trigger the onset of OSA (e.g., stroke). Care of these persons should be managed as clinically appropriate. This recommendation also does not apply to children, adolescents, or pregnant women.
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENT
Potential Preventable Burden. Based on data from the 1990s, the estimated prevalence of OSA in the United States is 10% for mild OSA and 3.8% to 6.5% for moderate to severe OSA.1–3 Current prevalence may be higher, given the increasing prevalence of obesity.4,5 Extrapolation from long-term follow-up data from the Wisconsin Sleep Cohort Study (1988–1994 to 2007–2010) results in an estimated prevalence of 16% for mild OSA and 10% for moderate to severe OSA.4 The prevalence of severe OSA in asymptomatic persons is unknown. In the Wisconsin Sleep Cohort Study, approximately 6% of adults with no or mild OSA progressed to moderate to severe OSA over 4 years.7
Risk factors associated with OSA include male sex, older age (40 to 70 years), postmenopausal status, higher BMI, and craniofacial and upper airway abnormalities. The evidence on other risk factors, such as smoking, alcohol and sedative use, and nasal congestion, is sparse or mixed.1
Observational studies have reported an association between severe OSA and mortality risk.8 In theory, screening for OSA could improve mortality by identifying OSA early and providing treatment before it can adversely influence mortality. Although studies generally show that treatment of OSA with CPAP and MADs improves intermediate outcomes, such as AHI and ESS score, there is a lack of studies demonstrating that change in AHI or ESS score improves health outcomes, and no well-controlled trials have demonstrated an improvement in mortality with treatment of OSA.
In trials reviewed by the USPSTF, treatment with CPAP effectively reduced AHI to normal (< 5) or near-normal (< 10) levels. Treatment with MADs showed more modest improvements in AHI. Treatment with either CPAP or MADs improved ESS scores by approximately 2 points, and trials evaluating treatment with CPAP also found reductions in blood pressure. However, the clinical significance of these small reductions is unclear. Of note, trials that evaluated treatment with CPAP or MADs were primarily conducted in referred or sleep clinic patients, not screen-detected patients from primary care settings.
Potential Harms. Direct evidence on the harms of screening for OSA is lacking. Commonly reported harms of treatment with CPAP include oral or nasal dryness, eye or skin irritation, rash, epistaxis, and pain.1 An estimated 14% to 32% of patients discontinue treatment with CPAP over 4 years.6 Commonly reported harms of treatment with MADs include oral mucosal, dental, or jaw symptoms, such as mucosal or dental pain, discomfort or tenderness, mucosal erosions, and jaw or temporomandibular joint pain or discomfort. Less common harms include oral dryness and excess salivation. Limited study data suggest that 7% of patients discontinue treatment with MADs because of harms.1
Current Practice. Most primary care clinicians do not routinely screen for OSA.1 According to a practice-based research network study of 44 practices, only 20% of patients with sleep-related symptoms who regularly visit a primary care clinician spontaneously reported their symptoms to their clinician.9 Some potential barriers to screening cited by clinicians include being unsure about how to identify and diagnose OSA, uncertainty regarding which type of sleep monitors are best for the diagnosis of OSA, and how to follow up patients who have been diagnosed with OSA.1
SCREENING TESTS
Potential screening questionnaires and clinical prediction tools include the ESS, STOP Questionnaire (Snoring, Tiredness, Observed Apnea, High Blood Pressure), STOP-Bang Questionnaire (STOP Questionnaire plus BMI, Age, Neck Circumference, and Gender), Berlin Questionnaire, Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction tool. However, none of these instruments have been adequately validated in a primary care setting.1
This recommendation statement was first published in JAMA. 2017;317(4):407–414.
The “Other Considerations,” “Discussion,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
