
Am Fam Physician. 2017;96(2):101-107
Author disclosure: No relevant financial affiliations.
Nearly 25% of U.S. adults report concurrently taking a prescription medication with a dietary supplement. Some supplements, such as St. John's wort and goldenseal, are known to cause clinically important drug interactions and should be avoided by most patients receiving any pharmacologic therapy. However, many other supplements are predicted to cause interactions based only on in vitro studies that have not been confirmed or have been refuted in human clinical trials. Some supplements may cause interactions with a few medications but are likely to be safe with other medications (e.g., curcumin, echinacea, garlic, Asian ginseng, green tea extract, kava kava). Some supplements have a low likelihood of drug interactions and, with certain caveats, can safely be taken with most medications (e.g., black cohosh, cranberry, ginkgo, milk thistle, American ginseng, saw palmetto, valerian). Clinicians should consult reliable dietary supplement resources, or clinical pharmacists or pharmacologists, to help assess the safety of specific herbal supplement–drug combinations. Because most patients do not disclose supplement use to clinicians, the most important strategy for detecting herb-drug interactions is to develop a trusting relationship that encourages patients to discuss their dietary supplement use.
Estimates show that between 40% and 60% of U.S. adults with chronic disease use dietary supplements, and among patients taking prescription medications, an estimated 20% to 25% concurrently use a dietary supplement.1–3 Accordingly, there has been increasing concern over the potential for dietary supplements, particularly herbal dietary supplements, to interact with prescription medications. The National Center for Complementary and Integrative Health defines dietary supplements as a variety of products, including herbs, vitamins and minerals, and probiotics. This review focuses on drug interactions with herbal dietary supplements, which are defined as supplements containing whole plant or plant extracts that are consumed as powder, capsule, tablet, or liquid formulations.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Clinicians should ask patients who take over-the-counter or prescription medications about their use of dietary supplements. | C | 5, 46, 47 | Many patients use dietary supplements, yet few discuss supplement use with their physicians. |
| Drug interactions with the following herbal supplements are unlikely or very limited: black cohosh, cranberry, ginkgo, American ginseng, milk thistle, saw palmetto, and valerian. With specific exceptions (e.g., ginkgo and warfarin [Coumadin]), clinicians should have low concern for drug interactions with these herbal supplements. | C | 7–11, 13–17, 22, 24, 27–30, 41–43, 45 | Numerous clinical trials report consistent findings. |
| Drug interactions with goldenseal and St. John's wort are highly likely, and clinicians should counsel patients to avoid concurrent use with over-the-counter or prescription medications. | C | 12, 13, 15, 23, 44 | Several clinical trials report consistent findings. |
Pharmacokinetic vs. Pharmacodynamic Interactions
Clinically important interactions between an herbal supplement and a drug typically manifest as pharmacokinetic interactions, which affect a drug's concentration in the blood and pharmacologic action. In many cases, pharmacokinetic interactions can be safely countered by adjusting the drug's dosage. Risk of a pharmacokinetic interaction occurs when an herbal supplement shares the same mechanism of absorption, distribution, metabolism, or excretion (ADME) as a coadministered drug. Competition between an herbal supplement and a drug for a shared ADME mechanism may result in a change in the drug's concentration at the site of action.
Less commonly, herb-drug interactions may manifest as pharmacodynamic interactions, which involve direct pharmacologic actions of an herbal supplement that are unrelated to changes in blood concentrations. Risk of a pharmacodynamic interaction occurs when an herbal supplement has a direct effect on the mechanism of action of a coadministered drug. Direct pharmacologic effects of an herbal supplement may antagonize or exacerbate the drug's clinical effects without changing the drug's concentration. In most cases, a change in drug dosage will not counter a pharmacodynamic herb-drug interaction.
Clinicians who are able to distinguish between pharmacokinetic/ADME interactions and pharmacodynamic interactions will be able to make better clinical decisions about whether to adjust the drug's dosage or discontinue the supplement. A change in drug dosage rarely results in a predictable change in clinical outcomes when a pharmacodynamic interaction occurs (e.g., warfarin [Coumadin] dosage adjustment with changes in daily leafy green vegetable intake).
Knowledge of the mechanisms underlying ADME processes for a drug and an herbal supplement is needed to recognize pharmacokinetic herb-drug interactions. Most ADME mechanisms fall under four large gene superfamilies comprising more than 1,000 proteins: the cytochrome P450 (CYP) drug metabolism enzymes; the uridine diphosphate-glucuronosyltransferase (UGT) conjugating enzymes; the adenosine triphosphate–binding cassette (ABC) drug uptake/efflux transporters; and the organic anion-transporting polypeptide (OATP) drug transporters. CYP and UGT enzymes make drugs more water-soluble, thus easier to eliminate from the body, whereas the ABC transporters (e.g., efflux pump P-glycoprotein [P-gp]) and OATP transporters are responsible for transporting drugs and their metabolites from various body compartments. This article centers on herb-drug interactions involving the ADME proteins that are most well characterized to date (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, OATP1A1, OATP1A2, OATP2B1, and P-gp). Importantly, the six CYP enzymes account for the metabolism of approximately 80% of all prescribed drugs.4
Drug interactions are initially evaluated through in vitro systems. Although in vitro evaluations have high sensitivity and can be used to rule out potential herb-drug interactions, it is important to follow up positive in vitro findings with a human clinical trial to estimate the potential impact of an interaction on clinical outcomes. Many positive in vitro interactions have not been borne out in human trials, highlighting the importance of confirming potential interactions.
Interaction Risks for Specific Herbal Supplements
This article summarizes 15 commonly used herbal dietary supplements based on national surveys and clinical experience.5,6 The list is not comprehensive; herbal supplements not included here may still have the potential for drug interactions. Although most of these supplements have information available on pharmacokinetic or pharmacodynamic interactions based on human clinical investigations, there are few supplements with clinical data that either consistently support important drug interactions or that clearly demonstrate no risk of interactions. Based on information from available studies, Table 1 lists supplements with a low risk of clinically important interactions,7–11 and Table 2 lists those with a high risk of clinically important interactions.12 Important caveats are noted in each table. A third group of supplements contains herbs that have shown a low risk of interaction with some drugs and a moderate or high risk of interaction with other drugs. Five additional supplements have insufficient or no data to categorize into one of these groups: acai (Euterpe oleracea), amalaki (Phyllanthus emblica), astragalus (Astragalus membranaceus), tongkat ali (Eurycoma longifolia), and Siberian ginseng (Eleutherococcus senticosus).
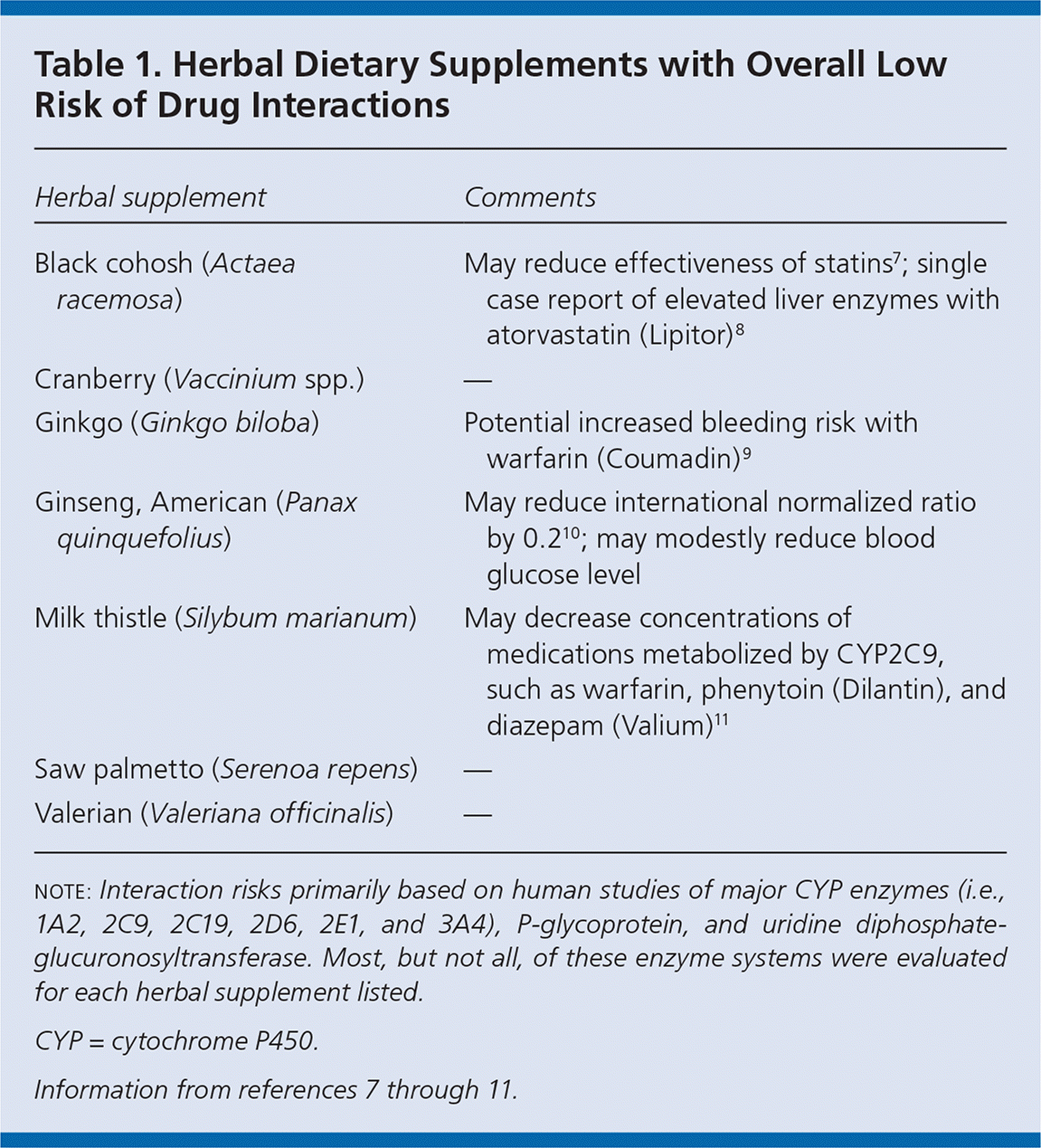
| Herbal supplement | Comments |
|---|---|
| Black cohosh (Actaea racemosa) | May reduce effectiveness of statins7; single case report of elevated liver enzymes with atorvastatin (Lipitor)8 |
| Cranberry (Vaccinium spp.) | — |
| Ginkgo (Ginkgo biloba) | Potential increased bleeding risk with warfarin (Coumadin)9 |
| Ginseng, American (Panax quinquefolius) | May reduce international normalized ratio by 0.210; may modestly reduce blood glucose level |
| Milk thistle (Silybum marianum) | May decrease concentrations of medications metabolized by CYP2C9, such as warfarin, phenytoin (Dilantin), and diazepam (Valium)11 |
| Saw palmetto (Serenoa repens) | — |
| Valerian (Valeriana officinalis) | — |
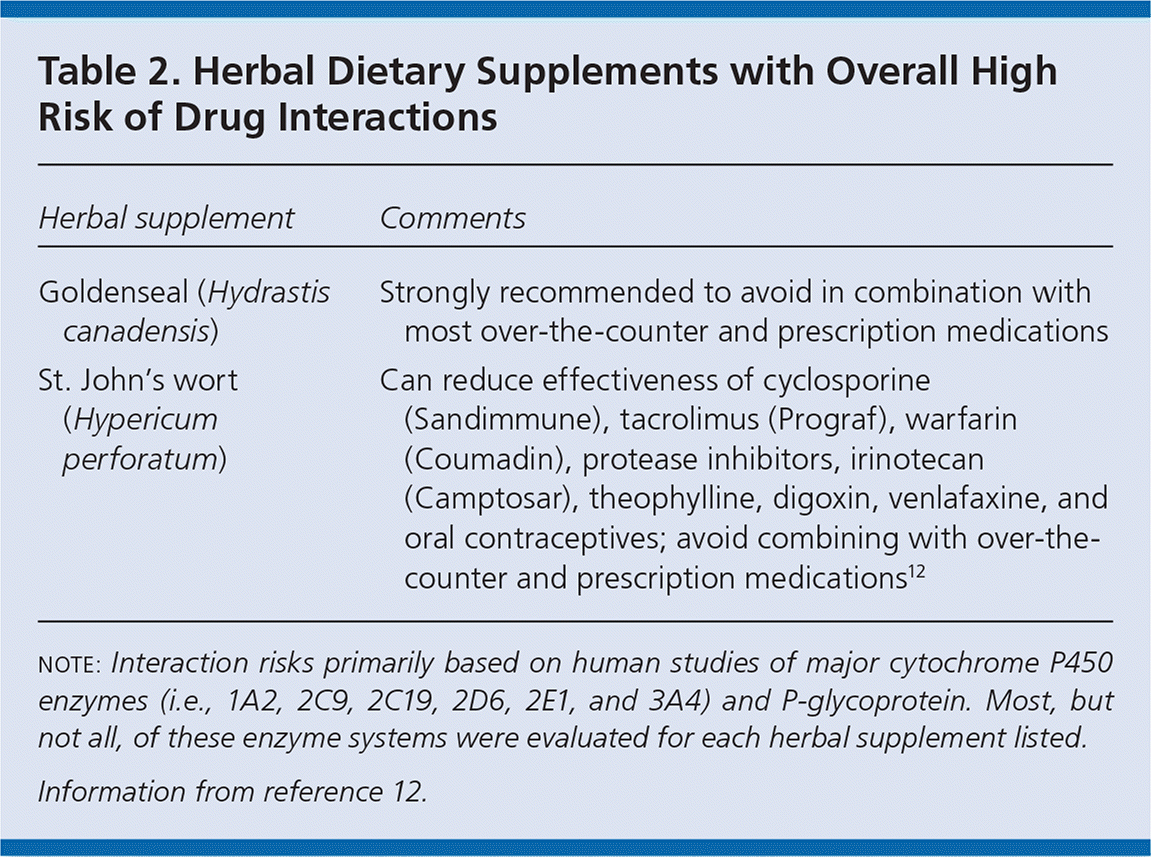
| Herbal supplement | Comments |
|---|---|
| Goldenseal (Hydrastis canadensis) | Strongly recommended to avoid in combination with most over-the-counter and prescription medications |
| St. John's wort (Hypericum perforatum) | Can reduce effectiveness of cyclosporine (Sandimmune), tacrolimus (Prograf), warfarin (Coumadin), protease inhibitors, irinotecan (Camptosar), theophylline, digoxin, venlafaxine, and oral contraceptives; avoid combining with over-the-counter and prescription medications12 |
BLACK COHOSH
Black cohosh (Actaea racemosa, formerly Cimicifuga racemosa) has been shown in several human clinical trials to have no clinically important effects on multiple CYP enzymes and P-gp.13–15 However, there is potential concern for interactions with OATP2B1, which could reduce the effectiveness of such drugs as amiodarone, fexofenadine (Allegra), glyburide, and many statin medications.7
CRANBERRY
In a human clinical trial, cranberry (Vaccinium spp.) has been shown to have no inhibitory or induction effects on the drug-metabolizing enzymes CYP1A2, CYP2C9, and CYP3A4.16 Additionally, despite anecdotal case reports of cranberry increasing warfarin concentrations and international normalized ratio (INR), two human clinical trials did not show a significant effect on either outcome.17 As a result, the likelihood that cranberry has any clinically important drug interactions is low.
CURCUMIN
A single study showed that curcumin (Curcuma longa) induces CYP1A2, which could cause decreased levels of many antidepressant and antipsychotic medications.18 It has also been shown to increase sulfasalazine (Azulfidine) levels.19 However, a few human clinical trials have demonstrated no effect on several important enzymes, including CYP2C9, CYP3A4, and UGT.20 Given these differences in enzymatic effects, consultation with appropriate dietary supplement resources is needed to determine the potential for interaction between curcumin and many medications.
ECHINACEA
Echinacea (Echinacea purpurea) has shown no inhibitory or inductive effects on CYP2D6, CYP2C9, or P-gp in human studies.15,21–23 There are conflicting results, however, about effects on CYP1A2 and CYP3A4, potentially with even short-term use. For this reason, caution should be taken when combining echinacea with medications that are metabolized by either of these CYP enzymes, including antipsychotic and antidepressant medications.21
GARLIC
Garlic (Allium sativum) extract has been shown in human studies to decrease concentrations of drugs that are transported by P-gp, but it has shown no effects on CYP1A2, CYP2D6, or CYP3A4.24–26 Medications that are transported by P-gp (e.g., colchicine, digoxin, doxorubicin [Adriamycin], quinidine, rosuvastatin [Crestor], tacrolimus [Prograf], verapamil) should not be combined with garlic supplements.
GINKGO
Ginkgo (Ginkgo biloba) is known to inhibit platelet aggregation, which could theoretically increase bleeding risk, especially in combination with antiplatelet or anticoagulant drugs. Several population-based and clinical studies, including a meta-analysis of 18 trials, failed to demonstrate that ginkgo increased bleeding risk or had significant effects on hematologic parameters.27 However, analysis of a single medical record database suggested an increased risk of bleeding with concurrent ginkgo and warfarin use.9 Patients taking warfarin should have their INR closely monitored or refrain from ginkgo use. Several human clinical trials have demonstrated no clinically important effects on CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4.24,28
GINSENG (AMERICAN)
American ginseng (Panax quinquefolius), although similar to its Asian counterpart (Panax ginseng), has a somewhat different profile of ginsenosides; therefore, generalizations about Asian ginseng should not be extended to American ginseng. There are fewer human clinical studies of American ginseng than Asian ginseng, although preclinical studies support a low potential for drug interactions. Two human trials have demonstrated no effect of American ginseng on the human immunodeficiency virus (HIV) agents indinavir (Crixivan) and zidovudine (Retrovir).29,30 A single trial of American ginseng in healthy volunteers taking warfarin demonstrated a 0.2-point drop in INR.10 Patients taking warfarin should have their INR closely monitored or refrain from taking ginseng-containing supplements.
GINSENG (ASIAN)
Asian ginseng (P. ginseng) has been shown in one study to induce CYP3A4, which could decrease the effectiveness of many drugs, including calcium channel blockers, many chemotherapy and HIV agents, certain antihypertensive and statin medications, and some antidepressants.31 For this reason, it is suggested to avoid use of Asian ginseng, including products containing Chinese, Japanese, and Korean ginseng, with most medications. However, several human trials have demonstrated that Asian ginseng has no effect on CYP1A2, CYP2D6, CYP2E1, or P-gp, so drugs metabolized by these enzymes may be safe to take concurrently with Asian ginseng.24,31,32 Studies of the effect of Asian ginseng on warfarin metabolism have yielded mixed results, alternately demonstrating no effect or small effects.33 In patients having difficulty maintaining adequate anticoagulation with warfarin, clinicians may suggest avoiding Asian ginseng products.
GOLDENSEAL
Goldenseal (Hydrastis canadensis) has been shown to inhibit two major metabolic enzymes, CYP2D6 and CYP3A4, which are responsible for metabolism of more than one-half of currently used pharmaceutical agents.13,15 Although some drug combinations with goldenseal may be safe, until data from further human clinical trials are available, clinicians should recommend against the use of goldenseal in combination with most other medications.
GREEN TEA EXTRACT
Green tea (Camellia sinensis) extract has been investigated for potential drug interactions in human and in vitro studies with conflicting results. In vitro study results have suggested potential interactions, whereas human clinical trials have not found any effects on the major metabolic enzymes CYP2D6 and CYP3A4.34 However, green tea extract has been shown to increase simvastatin (Zocor) concentrations,35 which may be due to P-gp inhibition. Two additional studies have demonstrated that green tea extract may inhibit the drug transporters OATP1A1 and OATP1A2, which are involved in the transport of many medications, including statins, fluoroquinolones, some beta blockers, imatinib (Gleevec), and antiretrovirals.36,37 Therefore, green tea extract should be avoided in combination with drugs that are transported by P-gp, OATP1A1, or OATP1A2.
KAVA KAVA
Kava kava (Piper methysticum) has been shown in multiple human studies to have no effect on CYP1A2, CYP2D6, CYP3A4, or P-gp.13,15,38,39 In one study of human volunteers, kava inhibited CYP2E1, which is involved in the metabolism of several anesthetic agents, as well as acetaminophen.13 Additionally, the results of two in vitro studies suggest the potential to inhibit CYP2C9 and CYP2C19, which are involved in the metabolism of many nonsteroidal anti-inflammatory drugs, angiotensin receptor blockers, glipizide (Glucotrol), glyburide, rosiglitazone (Avandia), valproic acid (Depakene), warfarin, proton pump inhibitors, phenytoin (Dilantin), and clopidogrel (Plavix).40 Patients taking kava should be counseled to stop at least five days before surgery with general anesthesia. Patients taking medications metabolized by CYP2C9 or CYP2C19 should be closely monitored for clinical adverse effects and laboratory abnormalities (e.g., glucose level, A1C level, INR) or instructed not to use kava-containing supplements. Caution should be exercised in patients using central nervous system depressants, such as benzodiazepines or alcohol, because of the increased risk of drowsiness and motor reflex depression.
MILK THISTLE
Milk thistle (Silybum marianum) does not have inhibitory or inductive effects on CYP1A2, CYP2D6, CYP2E1, CYP3A4, or P-gp, as demonstrated in multiple human studies.14,15,22,41,42 Milk thistle may reduce losartan (Cozaar) metabolism, depending on CYP2C9 genotype.11 There is potential for milk thistle to decrease concentrations of other medications metabolized by CYP2C9 such as warfarin, phenytoin, and diazepam (Valium).
SAW PALMETTO
ST. JOHN'S WORT
St. John's wort (Hypericum perforatum) has been shown in multiple human studies to be a potent inducer of CYP3A4 and P-gp.12,23,44 Clinical studies have shown reductions in cyclosporine (Sandimmune), tacrolimus, warfarin, protease inhibitors, irinotecan (Camptosar), theophylline, digoxin, venlafaxine, and oral contraceptives. It is strongly recommended to avoid concurrent use of St. John's wort with over-the-counter and prescription medications.
VALERIAN
Patient-Centered Approach to Dietary Supplement Use
It is paramount that clinicians have a continuing, open dialogue with patients about their use of dietary supplements. Several studies have shown that although more than 17% of Americans take dietary supplements, only one-third of them inform their physician.5,46 Although patients do not expect their physician to be an expert about dietary supplements, they prefer to have him or her initiate the conversation about supplement use.47 It is important for clinicians to use current resources because new data from in vitro and human herb-drug interaction studies are being published regularly. Older resources and those without periodic updates may present misleading or incorrect information and recommendations. Examples of reliable sources include PubMed, Natural Medicines database, the Allied and Complementary Medicine Database, Lexi-Natural Products, and the National Institutes of Health's Office of Dietary Supplements (Table 3).
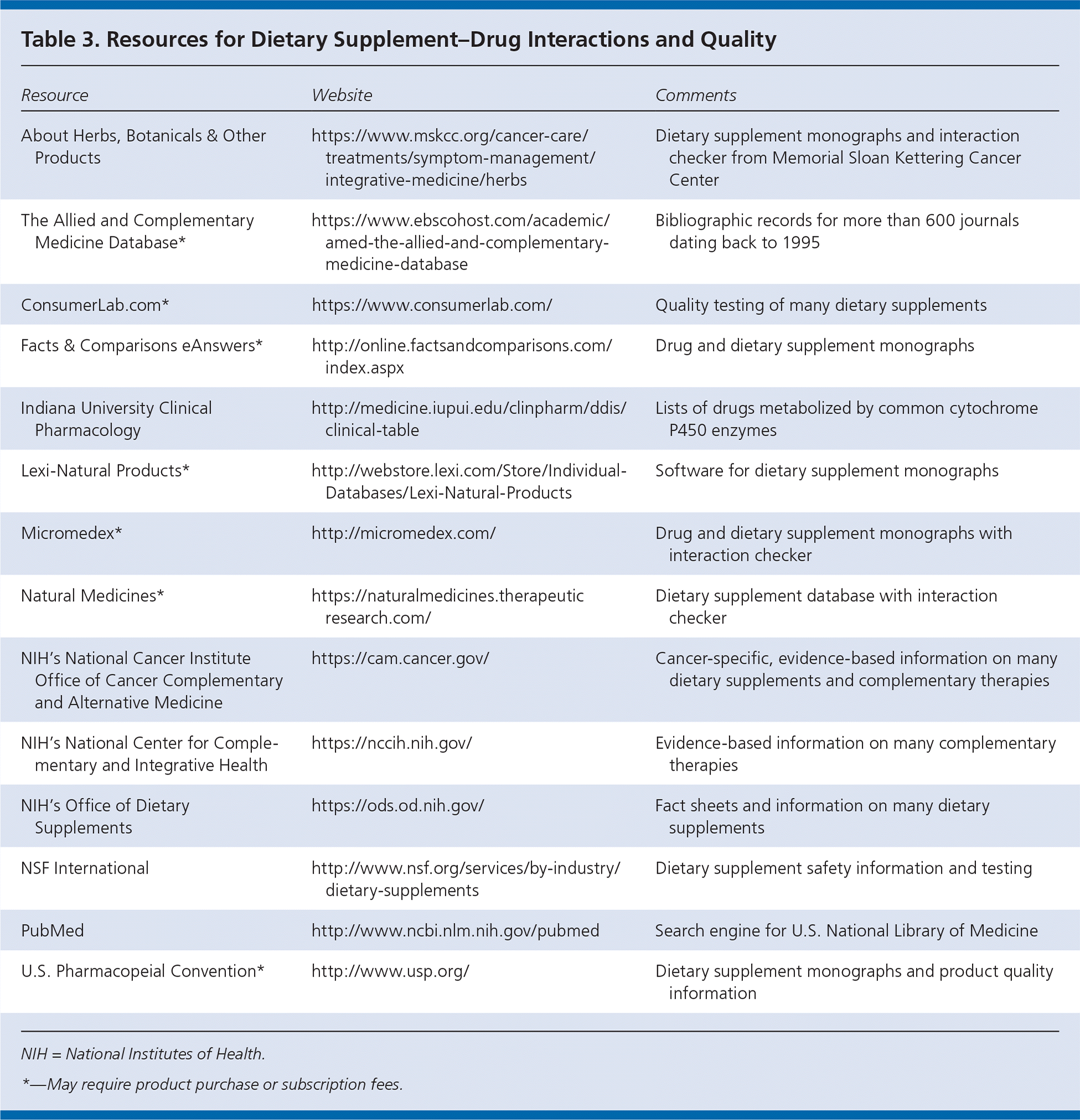
| Resource | Website | Comments |
|---|---|---|
| About Herbs, Botanicals & Other Products | https://www.mskcc.org/cancer-care/treatments/symptom-management/integrative-medicine/herbs | Dietary supplement monographs and interaction checker from Memorial Sloan Kettering Cancer Center |
| The Allied and Complementary Medicine Database* | https://www.ebscohost.com/academic/amed-the-allied-and-complementary-medicine-database | Bibliographic records for more than 600 journals dating back to 1995 |
| ConsumerLab.com* | https://www.consumerlab.com/ | Quality testing of many dietary supplements |
| Facts & Comparisons eAnswers* | http://online.factsandcomparisons.com/index.aspx | Drug and dietary supplement monographs |
| Indiana University Clinical Pharmacology | http://medicine.iupui.edu/clinpharm/ddis/clinical-table | Lists of drugs metabolized by common cytochrome P450 enzymes |
| Lexi-Natural Products* | http://webstore.lexi.com/Store/Individual-Databases/Lexi-Natural-Products | Software for dietary supplement monographs |
| Micromedex* | http://micromedex.com/ | Drug and dietary supplement monographs with interaction checker |
| Natural Medicines* | https://naturalmedicines.therapeuticresearch.com/ | Dietary supplement database with interaction checker |
| NIH's National Cancer Institute Office of Cancer Complementary and Alternative Medicine | https://cam.cancer.gov/ | Cancer-specific, evidence-based information on many dietary supplements and complementary therapies |
| NIH's National Center for Complementary and Integrative Health | https://nccih.nih.gov/ | Evidence-based information on many complementary therapies |
| NIH's Office of Dietary Supplements | https://ods.od.nih.gov/ | Fact sheets and information on many dietary supplements |
| NSF International | http://www.nsf.org/services/by-industry/dietary-supplements | Dietary supplement safety information and testing |
| PubMed | http://www.ncbi.nlm.nih.gov/pubmed | Search engine for U.S. National Library of Medicine |
| U.S. Pharmacopeial Convention* | http://www.usp.org/ | Dietary supplement monographs and product quality information |
For patients taking prescription medications, if few or no data are available on the potential for specific herb-drug interactions, the conservative approach is to recommend against supplement use. However, clinicians should recognize that these interactions are infrequent. Reliance on patient monitoring for adverse effects may be the best way to protect against adverse interactions if a drug has a wide therapeutic window, its clinical effects are readily monitored, and predetermined drug concentrations are not being targeted. When the evidence or recommendations are unclear, consultation with a clinical pharmacist or pharmacologist may be helpful.
Lastly, product quality is important. Reports of contaminants such as pesticides, heavy metals, and bacteria, as well as adulteration with prescription medications or other plant material, continue to surface. It can also be challenging to ensure that a supplement contains adequate concentrations of active components. The U.S. Food and Drug Administration's Current Good Manufacturing Practices are now in place for the supplement industry. Manufacturers should be able to provide a certificate of analysis for their products, which includes verification of active components in addition to concentrations of heavy metals and other potential contaminants. Resources such as U.S. Pharmacopeial Convention, ConsumerLab.com, and NSF International are helpful to identify manufacturers that produce high-quality dietary supplements (Table 3).
Data Sources: We generated a list of commonly used herbal dietary supplements based on national surveys of complementary and alternative medicine use, such as the National Health Interview Survey and the National Health and Nutrition Examination Survey, in addition to our own experience. We then searched PubMed using the MeSH term herb-drug interaction, and the common and/or botanical name of each dietary supplement. When no applicable information was available using that strategy, we expanded the terms to include the MeSH terms pharmacokinetics and pharmacodynamics. We sorted results into two categories: human clinical trials and in vitro/animal studies, and prioritized human studies. We reviewed available professional monographs for each supplement in the Natural Medicines database and the Facts & Comparisons eAnswers database for additional references, as well as Micromedex for additional drug information. Search dates: January 2016 and February 2017.
The authors thank Britnae Purdy, MA, for her assistance obtaining materials for this review.
