Am Fam Physician. 2017;96(3):online
Author disclosure: No relevant financial affiliations.

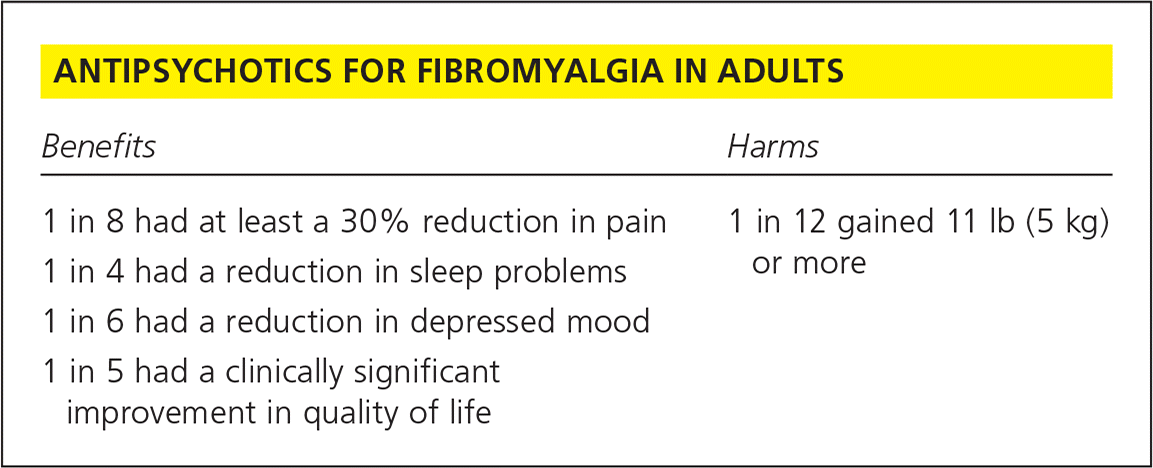
| Benefits | Harms |
|---|---|
| 1 in 8 had at least a 30% reduction in pain | 1 in 12 gained 11 lb (5 kg) or more |
| 1 in 4 had a reduction in sleep problems | |
| 1 in 6 had a reduction in depressed mood | |
| 1 in 5 had a clinically significant improvement in quality of life |
Details for This Review
Study Population: Adults with fibromyalgia
Efficacy End Points: At least 30% reduction in pain; reduction in depressed mood; reduction in sleep problems; clinically significant improvement in quality of life
Harm End Points: Any adverse event including weight gain and participants dropping out because of an adverse effect
Narrative: Fibromyalgia is characterized by chronic, widespread musculoskeletal pain, often associated with fatigue, memory problems, and sleep disturbances.1 However, ongoing research suggests it is a disorder of pain regulation or central sensitization.2 Up to 70% of patients with fibromyalgia also meet criteria for depression or anxiety, so medications for these conditions are often used to treat fibromyalgia.
This Cochrane review evaluated the evidence available for the use of antipsychotics, specifically quetiapine (Seroquel), to treat fibromyalgia.3 A total of four studies with 296 participants were reviewed. Three studies compared the use of quetiapine at bedtime with placebo (n = 206), and the fourth study (n = 90) compared quetiapine with amitriptyline. For the primary measured outcome of at least 50% reduction in pain, quetiapine was not statistically more effective than the control. For the secondary outcome, reduction of pain by at least 30%, two studies (n = 155) found a statistically significant benefit. Twenty of 82 participants (24.4%) receiving quetiapine and eight of 73 participants (11.0%) receiving a placebo reported pain relief of at least 30% (risk difference [RD] = 0.12%; 95% confidence interval [CI], 0.00 to 0.23; P = .04). The number needed to treat (NNT) for an additional person to benefit was 8 (95% CI, 5 to 100).
In two studies (n = 87), there was evidence that quetiapine was superior to placebo in reducing sleep problems, another secondary end point (standardized mean difference [SMD] = −0.67; 95% CI, −1.10 to −0.23; P = .003; NNT = 4).
Three studies (n = 207) were evaluated in an analysis of depression, a secondary end point. Quetiapine was superior to placebo in reducing depressed mood (SMD = −0.39; 95% CI, −0.74 to −0.04; P = .03; NNT = 6).
The final secondary end point was participant-reported improvement of health-related quality of life in the Fibromyalgia Impact Questionnaire (FIQ). Three studies (n = 206) were analyzed. The available evidence suggested that quetiapine was superior to placebo. Fifty-three of 107 participants (49.5%) in the quetiapine group and 32 of 99 participants (32.3%) in the placebo group reported a reduction of 14% or more of the FIQ total score (RD = 0.18; 95% CI, 0.05 to 0.31; P = .008; NNT = 5). A change in FIQ score of 14% has previously been defined as the minimal difference that is clinically relevant.4
Three studies with 206 participants were analyzed for safety and tolerability. The analysis showed very-low-quality evidence for no statistically significant difference between quetiapine and placebo in regard to withdrawal because of adverse events (RD = 0.10; 95% CI, −0.06 to 0.27; P = .21) and serious adverse events (RD = 0.00; 95% CI, −0.03 to 0.03; P = .96).
Two studies (n = 155) showed a statistically significant difference between quetiapine and placebo in weight gain. Seven of the 82 participants (8.5%) receiving quetiapine and none of the 73 participants receiving placebo reported a substantial weight gain (more than 11 lb [5 kg]; RD = 0.08; 95% CI, 0.02 to 0.15; P = .01.) The number needed to treat to harm was 12.
Caveats: The evidence reviewed was considered to be of generally low quality because of small sample sizes, imprecise results, and bias risk from limited study designs. Intended subgroup analyses, such as those with and without depression, could not be completed because of an insufficient number of studies. Quality of evidence was also downgraded because of indirectness (exclusions of patients with disease) and imprecision (fewer than 400 patients analyzed). Larger and more methodologically sound studies will be needed to verify the conclusions.
Quetiapine was the only antipsychotic the authors found that was studied in adults with fibromyalgia. Doses of quetiapine ranged from 50 to 300 mg at bedtime. The review concluded that quetiapine may be considered for a limited period (less than 12 weeks) to improve pain, sleep, and depression in fibromyalgia, while considering the potential for weight gain.
A reduction of at least 50% in pain is used by the Cochrane Pain, Palliative and Supportive Care Review Group as a threshold because it correlates with improvements in morbidity, function, and quality of life.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Contributing Editor, and Daniel Runde, MD, from the NNT Group (theNNT.com).
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/antipsychotics-fibromyalgia-adults/.