Am Fam Physician. 2017;96(3):170-178
Related editorial: Which Probiotics Should I Take? A Practical Guide for Family Physicians.
Patient information: See related handout on probiotics, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Probiotics contain microorganisms, most of which are bacteria similar to the beneficial bacteria that occur naturally in the human gut. Probiotics have been widely studied in a variety of gastrointestinal diseases. The most-studied species include Lactobacillus, Bifidobacterium, and Saccharomyces. However, a lack of clear guidelines on when to use probiotics and the most effective probiotic for different gastrointestinal conditions may be confusing for family physicians and their patients. Probiotics have an important role in the maintenance of immunologic equilibrium in the gastrointestinal tract through the direct interaction with immune cells. Probiotic effectiveness can be species-, dose-, and disease-specific, and the duration of therapy depends on the clinical indication. There is high-quality evidence that probiotics are effective for acute infectious diarrhea, antibiotic-associated diarrhea, Clostridium difficile–associated diarrhea, hepatic encephalopathy, ulcerative colitis, irritable bowel syndrome, functional gastrointestinal disorders, and necrotizing enterocolitis. Conversely, there is evidence that probiotics are not effective for acute pancreatitis and Crohn disease. Probiotics are safe for infants, children, adults, and older patients, but caution is advised in immunologically vulnerable populations.
Probiotics contain microorganisms, most of which are bacteria similar to the beneficial bacteria that occur naturally in the human gut. They are available over-the-counter (OTC) or by prescription and in a variety of forms such as capsules, packets, or food supplements. Although most probiotics are available without a prescription, there may be an advantage to patients with prescription drug coverage because probiotics may be a covered benefit. Probiotics have been widely studied in a variety of gastrointestinal (GI) diseases, and one in five Americans takes probiotics for digestive problems.1 The most studied probiotics for human use belong to the Lactobacillus, Bifidobacterium, or Saccharomyces species.2 This article focuses on probiotic use in infants, children, and adults with GI conditions, and it excludes probiotics for non-GI diseases.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Probiotic use reduces the risk of antibiotic-associated diarrhea in children and adults. | A | 10, 34, 35 |
| Probiotic use may reduce the incidence of Clostridium difficile–associated diarrhea. | B | 13, 14 |
| Probiotic use significantly reduces the risk of hepatic encephalopathy, but there is insufficient evidence regarding the effect on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. | B | 17, 38 |
| Probiotic use increases remission rates in adults with ulcerative colitis. | A | 19, 20 |
| Probiotic use improves abdominal pain and global symptom scores in children and adults with irritable bowel syndrome. | B | 21–23 |
| Probiotic use reduces the incidence of necrotizing enterocolitis and mortality in preterm infants. | A | 26, 27 |
| Probiotic use is ineffective for acute pancreatitis and Crohn disease. | B | 19, 42–45 |
Mechanism of Action
The intestinal microbiome is composed of microbes that reside in the gut and may be altered by diet, lifestyle, exposure to toxins, and antibiotic use.3 There is a relationship between disease, health, the immune system, and changes in the microbiota.3 Probiotics have an important role in the maintenance of immunologic equilibrium in the GI tract through direct interaction with immune cells.4 The microbiome diversity is likely important in health maintenance, and it is likely that broad-spectrum probiotics may increase the effectiveness of treatment. The mechanisms of action of probiotics are complex and likely differ by species (eTable A).

| Block the adhesion of pathogenic bacteria to the intestinal epithelium; produce inhibitory agentsA1 |
| Enhance the intestinal immune responseA2 |
| Maintain normal levels of short-chain fatty acidsA3 |
| Modulate immune system function, such as suppression of intestinal proinflammatory cytokinesA4 |
| Repair intestinal permeabilityA4 |
| Suppress the growth of pathogenic bacteria by directly binding to gram-negative bacteriaA5 |
| Upregulate intestinal electrolyte absorptionA2 |
Regulatory Issues
Probiotics are available in two main forms: food and dietary supplements. Dietary supplements are regulated by the U.S. Food and Drug Administration’s Center for Food Safety and Applied Nutrition.5 If the probiotics are considered to be drugs for therapeutic purposes, then the product is regulated by the U.S. Food and Drug Administration using Current Good Manufacturing Practices and Investigational New Drug approval processes.6,7 However, there is a need to address quality-control issues related to the manufacture of probiotics. Third-party testing data are available on some OTC probiotic products.8,9
Dosing, Duration, and Clinical Indications
A Cochrane review found that a dosage of 5 billion colony-forming units or greater per day was significantly more effective than a lower dosage.10 Physicians and patients are encouraged to use third-party analysis results when selecting an OTC product.8 Clinical effectiveness may be obtained from OTC products by increasing the number of capsules taken to obtain adequate dosages of colony-forming units. Probiotic effectiveness can be species-, dose-, and disease-specific.11 The duration of probiotic use depends on the clinical indication. A lack of clear guidelines on when to use probiotics and the most effective probiotic for different GI conditions is often confusing for family physicians and their patients. Table 1 lists GI conditions that may improve with probiotic use.8–10,12–27 Table 2 shows a grid of probiotic species studied according to GI condition, and Table 3 lists some probiotic products available in the United States.
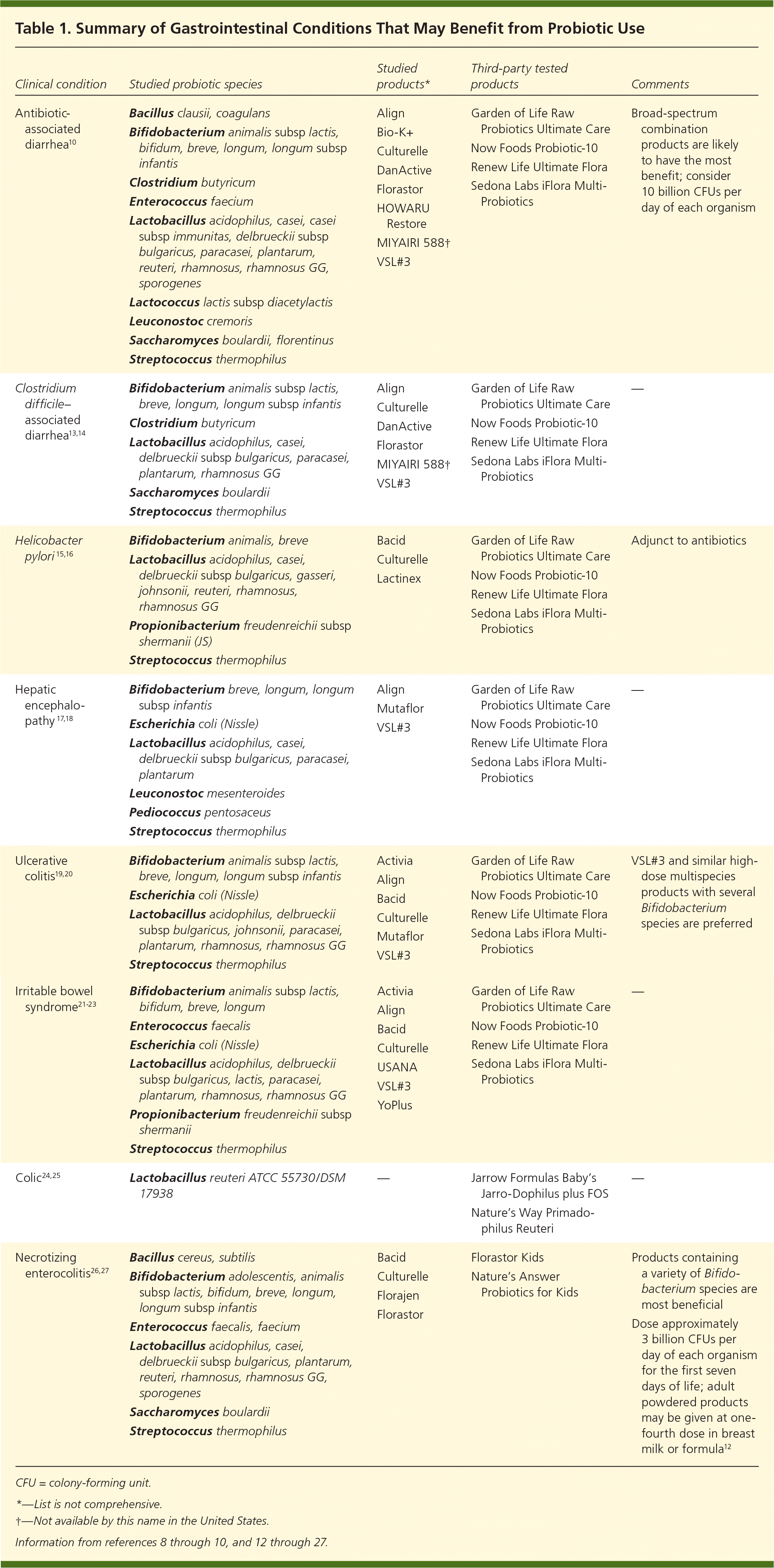
| Clinical condition | Studied probiotic species | Studied products* | Third-party tested products | Comments |
|---|---|---|---|---|
| Antibiotic-associated diarrhea10 | Bacillus clausii, coagulans | Align | Garden of Life Raw Probiotics Ultimate Care | Broad-spectrum combination products are likely to have the most benefit; consider 10 billion CFUs per day of each organism |
| Bifidobacterium animalis subsp lactis, bifidum, breve, longum, longum subsp infantis | Bio-K+ | Now Foods Probiotic-10 | ||
| Clostridium butyricum | Culturelle | Renew Life Ultimate Flora | ||
| Enterococcus faecium | DanActive | Sedona Labs iFlora Multi-Probiotics | ||
| Lactobacillus acidophilus, casei, casei subsp immunitas, delbrueckii subsp bulgaricus, paracasei, plantarum, reuteri, rhamnosus, rhamnosus GG, sporogenes | Florastor | |||
| Lactococcus lactis subsp diacetylactis | HOWARU Restore | |||
| Leuconostoc cremoris | MIYAIRI 588† | |||
| Saccharomyces boulardii, florentinus | VSL#3 | |||
| Streptococcus thermophilus | ||||
| Clostridium difficile–associated diarrhea13,14 | Bifidobacterium animalis subsp lactis, breve, longum, longum subsp infantis | Align | Garden of Life Raw Probiotics Ultimate Care | — |
| Clostridium butyricum | Culturelle | Now Foods Probiotic-10 | ||
| Lactobacillus acidophilus, casei, delbrueckii subsp bulgaricus, paracasei, plantarum, rhamnosus GG | DanActive | Renew Life Ultimate Flora | ||
| Saccharomyces boulardii | Florastor | Sedona Labs iFlora Multi-Probiotics | ||
| Streptococcus thermophilus | MIYAIRI 588† | |||
| VSL#3 | ||||
| Helicobacter pylori15,16 | Bifidobacterium animalis, breve | Bacid | Garden of Life Raw Probiotics Ultimate Care | Adjunct to antibiotics |
| Lactobacillus acidophilus, casei, delbrueckii subsp bulgaricus, gasseri, johnsonii, reuteri, rhamnosus, rhamnosus GG | Culturelle | Now Foods Probiotic-10 | ||
| Propionibacterium freudenreichii subsp shermanii (JS) | Lactinex | Renew Life Ultimate Flora | ||
| Streptococcus thermophilus | Sedona Labs iFlora Multi-Probiotics | |||
| Hepatic encephalopathy 17,18 | Bifidobacterium breve, longum, longum subsp infantis | Align | Garden of Life Raw Probiotics Ultimate Care | — |
| Escherichia coli (Nissle) | Mutaflor | Now Foods Probiotic-10 | ||
| Lactobacillus acidophilus, casei, delbrueckii subsp bulgaricus, paracasei, plantarum | VSL#3 | Renew Life Ultimate Flora | ||
| Leuconostoc mesenteroides | Sedona Labs iFlora Multi-Probiotics | |||
| Pediococcus pentosaceus | ||||
| Streptococcus thermophilus | ||||
| Ulcerative colitis19,20 | Bifidobacterium animalis subsp lactis, breve, longum, longum subsp infantis | Activia | Garden of Life Raw Probiotics Ultimate Care | VSL#3 and similar high-dose multispecies products with several Bifidobacterium species are preferred |
| Escherichia coli (Nissle) | Align | Now Foods Probiotic-10 | ||
| Lactobacillus acidophilus, delbrueckii subsp bulgaricus, johnsonii, paracasei, plantarum, rhamnosus, rhamnosus GG | Bacid | Renew Life Ultimate Flora | ||
| Streptococcus thermophilus | Culturelle | Sedona Labs iFlora Multi-Probiotics | ||
| Mutaflor | ||||
| VSL#3 | ||||
| Irritable bowel syndrome21–23 | Bifidobacterium animalis subsp lactis, bifidum, breve, longum | Activia | Garden of Life Raw Probiotics Ultimate Care | — |
| Enterococcus faecalis | Align | Now Foods Probiotic-10 | ||
| Escherichia coli (Nissle) | Bacid | Renew Life Ultimate Flora | ||
| Lactobacillus acidophilus, delbrueckii subsp bulgaricus, lactis, paracasei, plantarum, rhamnosus, rhamnosus GG | Culturelle | Sedona Labs iFlora Multi-Probiotics | ||
| Propionibacterium freudenreichii subsp shermanii | USANA | |||
| Streptococcus thermophilus | VSL#3 | |||
| YoPlus | ||||
| Colic24,25 | Lactobacillus reuteri ATCC 55730/DSM 17938 | — | Jarrow Formulas Baby’s Jarro-Dophilus plus FOS | — |
| Nature’s Way Primadophilus Reuteri | ||||
| Necrotizing enterocolitis26,27 | Bacillus cereus, subtilis | Bacid | Florastor Kids | Products containing a variety of Bifidobacterium species are most beneficial |
| Bifidobacterium adolescentis, animalis subsp lactis, bifidum, breve, longum, longum subsp infantis | Culturelle | Nature’s Answer Probiotics for Kids | Dose approximately 3 billion CFUs per day of each organism for the first seven days of life; adult powdered products may be given at one-fourth dose in breast milk or formula12 | |
| Enterococcus faecalis, faecium | Florajen | |||
| Lactobacillus acidophilus, casei, delbrueckii subsp bulgaricus, plantarum, reuteri, rhamnosus, rhamnosus GG, sporogenes | Florastor | |||
| Saccharomyces boulardii | ||||
| Streptococcus thermophilus |
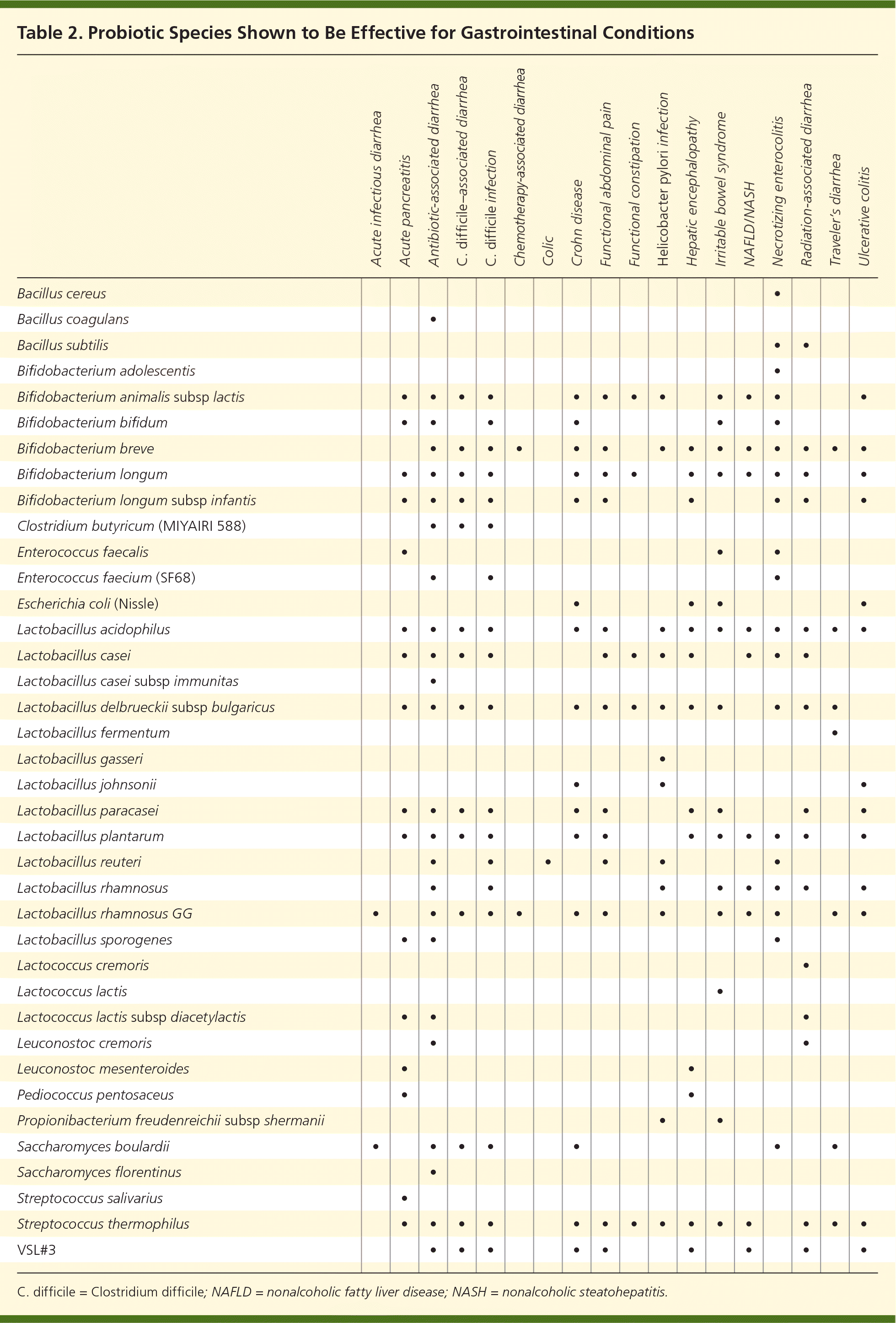
| Acute infectious diarrhea | Acute pancreatitis | Antibiotic-associated diarrhea | C. difficile–associated diarrhea | C. difficile infection | Chemotherapy-associated diarrhea | Colic | Crohn disease | Functional abdominal pain | Functional constipation | Helicobacter pylori infection | Hepatic encephalopathy | Irritable bowel syndrome | NAFLD/NASH | Necrotizing enterocolitis | Radiation-associated diarrhea | Traveler’s diarrhea | Ulcerative colitis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus cereus | • | |||||||||||||||||
| Bacillus coagulans | • | |||||||||||||||||
| Bacillus subtilis | • | • | ||||||||||||||||
| Bifidobacterium adolescentis | • | |||||||||||||||||
| Bifidobacterium animalis subsp lactis | • | • | • | • | • | • | • | • | • | • | • | • | ||||||
| Bifidobacterium bifidum | • | • | • | • | • | • | ||||||||||||
| Bifidobacterium breve | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||
| Bifidobacterium longum | • | • | • | • | • | • | • | • | • | • | • | • | • | |||||
| Bifidobacterium longum subsp infantis | • | • | • | • | • | • | • | • | • | • | ||||||||
| Clostridium butyricum (MIYAIRI 588) | • | • | • | |||||||||||||||
| Enterococcus faecalis | • | • | • | |||||||||||||||
| Enterococcus faecium (SF68) | • | • | • | |||||||||||||||
| Escherichia coli (Nissle) | • | • | • | • | ||||||||||||||
| Lactobacillus acidophilus | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||
| Lactobacillus casei | • | • | • | • | • | • | • | • | • | • | • | |||||||
| Lactobacillus casei subsp immunitas | • | |||||||||||||||||
| Lactobacillus delbrueckii subsp bulgaricus | • | • | • | • | • | • | • | • | • | • | • | • | • | |||||
| Lactobacillus fermentum | • | |||||||||||||||||
| Lactobacillus gasseri | • | |||||||||||||||||
| Lactobacillus johnsonii | • | • | • | |||||||||||||||
| Lactobacillus paracasei | • | • | • | • | • | • | • | • | • | • | ||||||||
| Lactobacillus plantarum | • | • | • | • | • | • | • | • | • | • | • | • | ||||||
| Lactobacillus reuteri | • | • | • | • | • | • | ||||||||||||
| Lactobacillus rhamnosus | • | • | • | • | • | • | • | • | ||||||||||
| Lactobacillus rhamnosus GG | • | • | • | • | • | • | • | • | • | • | • | • | • | |||||
| Lactobacillus sporogenes | • | • | • | |||||||||||||||
| Lactococcus cremoris | • | |||||||||||||||||
| Lactococcus lactis | • | |||||||||||||||||
| Lactococcus lactis subsp diacetylactis | • | • | • | |||||||||||||||
| Leuconostoc cremoris | • | • | ||||||||||||||||
| Leuconostoc mesenteroides | • | • | ||||||||||||||||
| Pediococcus pentosaceus | • | • | ||||||||||||||||
| Propionibacterium freudenreichii subsp shermanii | • | • | ||||||||||||||||
| Saccharomyces boulardii | • | • | • | • | • | • | • | |||||||||||
| Saccharomyces florentinus | • | |||||||||||||||||
| Streptococcus salivarius | • | |||||||||||||||||
| Streptococcus thermophilus | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||
| VSL#3 | • | • | • | • | • | • | • | • | • |
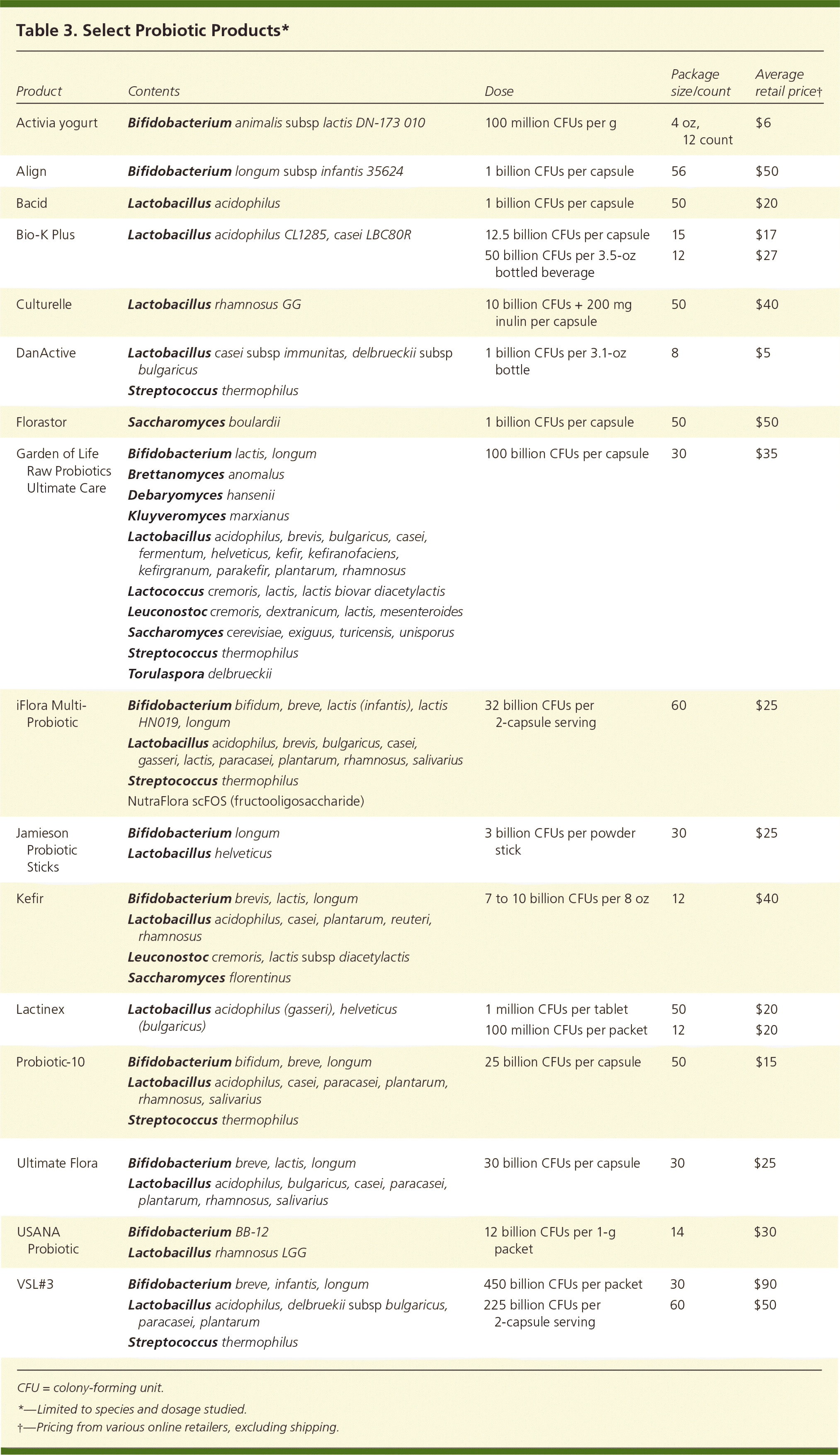
| Product | Contents | Dose | Package size/count | Average retail price† |
|---|---|---|---|---|
| Activia yogurt | Bifidobacterium animalis subsp lactis DN-173 010 | 100 million CFUs per g | 4 oz, 12 count | $6 |
| Align | Bifidobacterium longum subsp infantis 35624 | 1 billion CFUs per capsule | 56 | $50 |
| Bacid | Lactobacillus acidophilus | 1 billion CFUs per capsule | 50 | $20 |
| Bio-K Plus | Lactobacillus acidophilus CL1285, casei LBC80R | 12.5 billion CFUs per capsule | 15 | $17 |
| 50 billion CFUs per 3.5-oz bottled beverage | 12 | $27 | ||
| Culturelle | Lactobacillus rhamnosus GG | 10 billion CFUs + 200 mg inulin per capsule | 50 | $40 |
| DanActive | Lactobacillus casei subsp immunitas, delbrueckii subsp bulgaricus | 1 billion CFUs per 3.1-oz bottle | 8 | $5 |
| Streptococcus thermophilus | ||||
| Florastor | Saccharomyces boulardii | 1 billion CFUs per capsule | 50 | $50 |
| Garden of Life Raw Probiotics Ultimate Care | Bifidobacterium lactis, longum | 100 billion CFUs per capsule | 30 | $35 |
| Brettanomyces anomalus | ||||
| Debaryomyces hansenii | ||||
| Kluyveromyces marxianus | ||||
| Lactobacillus acidophilus, brevis, bulgaricus, casei, fermentum, helveticus, kefir, kefiranofaciens, kefirgranum, parakefir, plantarum, rhamnosus | ||||
| Lactococcus cremoris, lactis, lactis biovar diacetylactis | ||||
| Leuconostoc cremoris, dextranicum, lactis, mesenteroides | ||||
| Saccharomyces cerevisiae, exiguus, turicensis, unisporus | ||||
| Streptococcus thermophilus | ||||
| Torulaspora delbrueckii | ||||
| iFlora Multi-Probiotic | Bifidobacterium bifidum, breve, lactis (infantis), lactis HN019, longum | 32 billion CFUs per 2-capsule serving | 60 | $25 |
| Lactobacillus acidophilus, brevis, bulgaricus, casei, gasseri, lactis, paracasei, plantarum, rhamnosus, salivarius | ||||
| Streptococcus thermophilus NutraFlora scFOS (fructooligosaccharide) | ||||
| Jamieson Probiotic Sticks | Bifidobacterium longum | 3 billion CFUs per powder stick | 30 | $25 |
| Lactobacillus helveticus | ||||
| Kefir | Bifidobacterium brevis, lactis, longum | 7 to 10 billion CFUs per 8 oz | 12 | $40 |
| Lactobacillus acidophilus, casei, plantarum, reuteri, rhamnosus | ||||
| Leuconostoc cremoris, lactis subsp diacetylactis | ||||
| Saccharomyces florentinus | ||||
| Lactinex | Lactobacillus acidophilus (gasseri), helveticus (bulgaricus) | 1 million CFUs per tablet | 50 | $20 |
| 100 million CFUs per packet | 12 | $20 | ||
| Probiotic-10 | Bifidobacterium bifidum, breve, longum | 25 billion CFUs per capsule | 50 | $15 |
| Lactobacillus acidophilus, casei, paracasei, plantarum, rhamnosus, salivarius | ||||
| Streptococcus thermophilus | ||||
| Ultimate Flora | Bifidobacterium breve, lactis, longum | 30 billion CFUs per capsule | 30 | $25 |
| Lactobacillus acidophilus, bulgaricus, casei, paracasei, plantarum, rhamnosus, salivarius | ||||
| USANA Probiotic | Bifidobacterium BB-12 | 12 billion CFUs per 1-g packet | 14 | $30 |
| Lactobacillus rhamnosus LGG | ||||
| VSL#3 | Bifidobacterium breve, infantis, longum | 450 billion CFUs per packet | 30 | $90 |
| Lactobacillus acidophilus, delbruekii subsp bulgaricus, paracasei, plantarum | 225 billion CFUs per 2-capsule serving | 60 | $50 | |
| Streptococcus thermophilus |
Acute Infectious Diarrhea
Probiotics are effective for acute infectious diarrhea caused by bacteria, but there are inconsistent results for the effectiveness of probiotics for diarrhea caused by viruses. A Cochrane review of 63 randomized controlled trials (RCTs) and quasi-RCTs included 8,014 infants, children, and adults with acute infectious diarrhea. The researchers found that probiotics significantly reduced the mean duration of diarrhea (25 fewer hours; 95% confidence interval [CI], 16 to 34 fewer hours); decreased the risk of diarrhea lasting four or more days by 59%; and led to approximately one fewer stool on day 2 (mean difference = 0.80; 95% CI, 0.45 to 1.14).28 For patients with acute infectious diarrhea, probiotics should be started at the onset of symptoms and, although there is no evidence to support length of treatment, we suggest continuing for one to two weeks following the resolution of symptoms. A meta-analysis of 12 RCTs with 5,171 participants found a 15% relative decrease in the risk of traveler’s diarrhea with probiotic use (relative risk [RR] = 0.85; 95% CI, 0.79 to 0.91).29 For prevention of traveler’s diarrhea, probiotics should be started two days before travel and continued throughout the trip.
A meta-analysis of 17 RCTs in 2,102 children comparing probiotics vs. control for the treatment of acute diarrhea showed a significant reduction in the duration of diarrhea with probiotic use (20 fewer hours; 95% CI, 13 to 26 fewer hours).30 Another meta-analysis of eight RCTs involving 1,229 children found that Lactobacillus reuteri administration reduced the duration of diarrhea (25 fewer hours; 95% CI, 11 to 39 fewer hours) and increased the cure rate on days 1 and 2.31 However, an RCT of 646 children with acute watery diarrhea caused predominantly by rotavirus found no significant difference between the group that received Lactobacillus rhamnosus GG probiotics and the control group in the daily frequency of stools, duration of diarrhea, vomiting, or length of hospital stay.32 A meta-analysis of two RCTs in 201 children with diarrhea from rotavirus found a significant reduction in diarrhea in those treated with L. rhamnosus GG vs. placebo (two fewer days; 95% CI, 0.6 to 3.6 fewer days).33
Antibiotic-Associated Diarrhea, C. difficile Infection, and C. difficile–Associated Diarrhea
Probiotics are effective for the prevention and treatment of antibiotic-associated diarrhea in children and adults, and the prevention of Clostridium difficile–associated diarrhea in children and adults; however, there are conflicting results for C. difficile infection. Patients should start probiotics on the first day of antibiotic treatment and continue for one to two weeks following completion of antibiotic therapy. To simplify the treatment regimen, patients may take probiotics at the same time as antibiotics. A Cochrane review of probiotics for the prevention of antibiotic-associated diarrhea in children (23 studies with 3,938 participants) reported that children treated with probiotics vs. control were less likely to have antibiotic-associated diarrhea (absolute risk reduction [ARR] = 11%; number needed to treat [NNT] = 10).10 This review found that L. rhamnosus or Saccharomyces boulardii at 5 to 40 billion colony-forming units per day was effective, with rare adverse events. In an RCT of 333 hospitalized children receiving antibiotics, diarrhea prevalence was lower in children receiving S. boulardii probiotics compared with oral rehydration (ARR = 21%; NNT = 5). There was also a reduced risk of antibiotic-associated diarrhea, including C. difficile–associated diarrhea and culture-negative diarrhea (ARR = 15%; NNT = 7), as well as significantly lower stool frequency, higher recovery rate, and shorter mean duration of diarrhea (2.3 vs. 9.0 days; P < .001).34
A meta-analysis of 63 RCTs with 11,811 children and adults that compared probiotics with placebo or no treatment reported a significant reduction in the risk of antibiotic-associated diarrhea (NNT = 13).35 A meta-analysis of adult inpatients showed a reduction in antibiotic-associated diarrhea (15 RCTs; 2,296 patients; NNT = 11) and a reduction in C. difficile infection (nine RCTs; 1,099 patients; NNT = 14) among patients randomly assigned to probiotics vs. placebo.36 A Cochrane review of 23 RCTs reported that probiotics significantly reduced the risk of C. difficile–associated diarrhea vs. placebo (ARR = 3.5%; NNT = 29).13 This review did not find a significant difference in C. difficile infection with probiotics compared with placebo.13 A meta-analysis of 20 RCTs with 3,818 adults and children demonstrated a significantly decreased risk of C. difficile–associated diarrhea (NNT = 30).14 A meta-analysis of two low-quality RCTs including 495 children and adults found no significant effect of yogurt vs. placebo to prevent antibiotic-associated diarrhea.37
H. pylori Infection
There are inconsistent results on the effectiveness of probiotics as an adjunct to antibiotic therapy to improve Helicobacter pylori eradication rates. A meta-analysis of nine RCTs involving 1,163 children and adults found that using Lactobacillus-containing probiotics as an adjunct to antibiotics increased the H. pylori eradication rate compared with control (NNT = 10).15 However, a meta-analysis of 21 RCTs with 3,452 adults found that probiotics as an adjunct to antibiotics did not improve the eradication of H. pylori infection (odds ratio = 1.44; 95% CI, 0.87 to 2.39) compared with placebo.16
Hepatic Encephalopathy, Nonalcoholic Fatty Liver Disease, and Nonalcoholic Steatohepatitis
Although probiotics appear to be effective for hepatic encephalopathy, there is insufficient patient-oriented evidence of their effectiveness for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. A meta-analysis of six RCTs involving 496 adults with cirrhosis showed that probiotic therapy significantly reduced the development of overt hepatic encephalopathy (ARR = 15.3%; NNT = 7).17 A Cochrane review of 21 trials involving 1,420 participants found no effect on all-cause mortality for probiotics vs. placebo or no treatment, although probiotics may improve recovery and quality of life compared with placebo or no treatment.18 A systematic review of three RCTs concluded that probiotics improved liver function in adults with nonalcoholic steatohepatitis and nonalcoholic fatty liver disease based on disease-oriented markers, but clear patient-oriented evidence is lacking.38
Ulcerative Colitis
Probiotics are effective in increasing remission rates in adults with ulcerative colitis but not in maintenance of remission. Probiotics should be started at the onset of an exacerbation of ulcerative colitis, and we suggest continuing for one to two weeks following resolution of symptoms. A meta-analysis of 23 RCTs with 1,763 adults found that probiotics significantly increased the remission rates in patients with active ulcerative colitis compared with placebo (ARR = 12.3%; NNT = 8).19 A Cochrane review of four studies involving 587 participants found no significant difference between probiotics and mesalamine for the maintenance of remission in ulcerative colitis.20
Irritable Bowel Syndrome and Functional Abdominal Pain
Probiotics are somewhat effective in children and adults with irritable bowel syndrome (IBS) and in children with functional abdominal pain. Patients should consider starting probiotics at the onset of symptoms and continue as needed for persistent symptoms. A guideline and meta-analysis of 23 trials involving 2,575 children and adults with IBS found that probiotics significantly improved global symptoms, bloating, and flatulence compared with placebo (NNT = 7), but the quality of studies was low.21 A meta-analysis of 21 RCTs involving 1,639 adults with IBS found that probiotics significantly improved overall symptom response (RR = 1.82; 95% CI, 1.27 to 2.60) and quality of life (standard mean difference = 0.29; 95% CI, 0.08 to 0.50) compared with placebo.22 A meta-analysis of children with IBS or functional abdominal pain found that probiotics increased the likelihood of treatment success compared with placebo (RR = 1.5; 95% CI, 1.22 to 1.84) and decreased abdominal pain intensity; however, there was no effect on abdominal pain frequency.23 Although probiotics are a promising and reasonable treatment option for IBS, the overall quality and quantity of evidence are relatively weak.
Constipation
Probiotics are effective for children and adults with constipation. Patients should start probiotics at the onset of symptoms and continue as symptoms persist. A meta-analysis of two trials including 165 adults with chronic idiopathic constipation reported a significant increase in the mean number of stools per week in patients treated with probiotics vs. placebo (mean increase = 1.5; 95% CI, 1.0 to 2.0).39 An RCT of 59 children with functional chronic intestinal constipation found significant improvements favoring Bifidobacterium-containing yogurt vs. standard yogurt for improving defecation frequency, pain with defecation, and abdominal pain.40
Colic
There are inconsistent results on the effectiveness of probiotics for the prevention and treatment of colic based on a systematic review (prevention: seven RCTs with 1,554 infants; treatment: two RCTs with 62 infants).24 A meta-analysis of three RCTs found that infants treated with L. reuteri had a reduced risk of crying time at 14 and 21 days compared with placebo (NNT = 2), but this was based on only three studies with 209 infants.25
Necrotizing Enterocolitis
In infants, probiotics effectively decrease the risk of necrotizing enterocolitis and mortality. Therapy should be started in those at risk of the condition and continue as long as the increased risk persists. A Cochrane review found that probiotics compared with control or placebo significantly reduced the risk of severe necrotizing enterocolitis (RR = 0.43; 95% CI, 0.33 to 0.56; 20 studies with 5,529 infants) and mortality (RR = 0.65; 95% CI, 0.52 to 0.81; 17 studies with 5,112 infants).26 A meta-analysis of 12 RCTs including 10,800 preterm infants receiving probiotics vs. control found a reduction in the incidence of necrotizing enterocolitis (RR = 0.55; 95% CI, 0.39 to 0.78) and mortality (RR = 0.72; 95% CI, 0.61 to 0.85).27
Conditions for Which Probiotics Are Ineffective
Probiotics are not effective for acute pancreatitis or Crohn disease. A meta-analysis of six RCTs including 536 adults with severe acute pancreatitis showed that probiotics compared with control did not significantly affect pancreatic infection rate, total number of infections, operation rate, hospital length of stay, or mortality.41 Three Cochrane reviews found insufficient evidence for the effectiveness of probiotics in patients with Crohn disease for induction of remission, maintenance of remission, or prevention of postoperative recurrence.19,42–44
Safety
Probiotics are generally considered safe but caution is advised in immunologically vulnerable populations. A systematic review by the Agency for Healthcare Research and Quality of 387 studies with a total of 24,615 participants did not find a significant increase in the number of adverse events in individuals treated with short-term probiotics (less than one month) based on 121 RCTs, or in the number of adverse-event incidents reported in probiotic vs. control groups based on 208 RCTs.45 The long-term effects of probiotics are largely unknown, and additional randomized trials are needed to address this question.45 This study found no significant increase in the risk of adverse events for children (35 RCTs), adults (40 RCTs), or older persons (four RCTs).45 However, a systematic review of 17 studies including 1,530 patients with cancer found five cases of probiotic-related bacteremia/fungemia/positive blood culture.46
This article updates a previous article on this topic by Kligler and Cohrssen.2
Data Sources: We completed a general PubMed search using the MeSH term probiotics. The term probiotics was also used in a number of specialized searches looking into specific topics in combination with one or more of the following terms: child, pediatric, adult, irritable bowel syndrome, irritable bowel disease, liver disease, prebiotics, diarrhea, functional abdominal pain, constipation, Clostridium difficile, Helicobacter pylori, colic, and necrotizing enterocolitis. The search included meta-analyses, randomized controlled trials, and practice guidelines within the previous 20 years. Also searched were the Cochrane databases and Essential Evidence Plus. Search dates: June and July 2015, and March 2017.
The authors thank Lindsay Blake for her assistance with the literature search.