
Am Fam Physician. 2017;96(7):472-473
Author disclosure: No relevant financial affiliations
Key Points for Practice
• Metformin should be the first medication prescribed for diabetes when an oral agent is required.
• Combination treatment is superior to metformin alone for decreasing A1C levels, weight, and blood pressure.
• Medications should be selected based on benefits, possible harms, and cost.
From the AFP Editors
Type 2 diabetes mellitus, which occurs in up to 95% of persons with diabetes, is typically managed with lifestyle modifications (e.g., diet, exercise) and medication (e.g., oral drugs). When weight loss or lifestyle modifications are initially unsuccessful, medication is often prescribed. The American College of Physicians (ACP) previously released guidelines in 2012 regarding the effectiveness and safety of oral pharmacologic treatment for type 2 diabetes; however, new evidence has emerged and new drugs have been approved by the U.S. Food and Drug Administration. For this reason, the ACP has released updated guidelines for the management of type 2 diabetes with oral medication.
Recommendations
If glycemic control needs to be improved with medication in persons with type 2 diabetes, metformin should be prescribed because it can efficiently lower glycemic levels, is linked to losing weight and fewer occurrences of hypoglycemia, and is generally less expensive than other options. It is contraindicated in persons with decreased tissue perfusion, hemodynamic instability, advanced liver disease, alcohol abuse, acute unstable congestive heart failure, and conditions that can result in lactic acidosis.
Because combination treatment has been shown to be superior to metformin alone for decreasing A1C levels, weight, and blood pressure, adding a sulfonylurea, thiazolidinedione, or sodium glucose cotransporter-2 (SGLT-2) or dipeptidyl peptidase-4 (DPP-4) inhibitor can be considered when additional oral treatment is being discussed. The choice of drug should be based on a conversation with the patient about benefits, possible harms, and cost (Table 1).
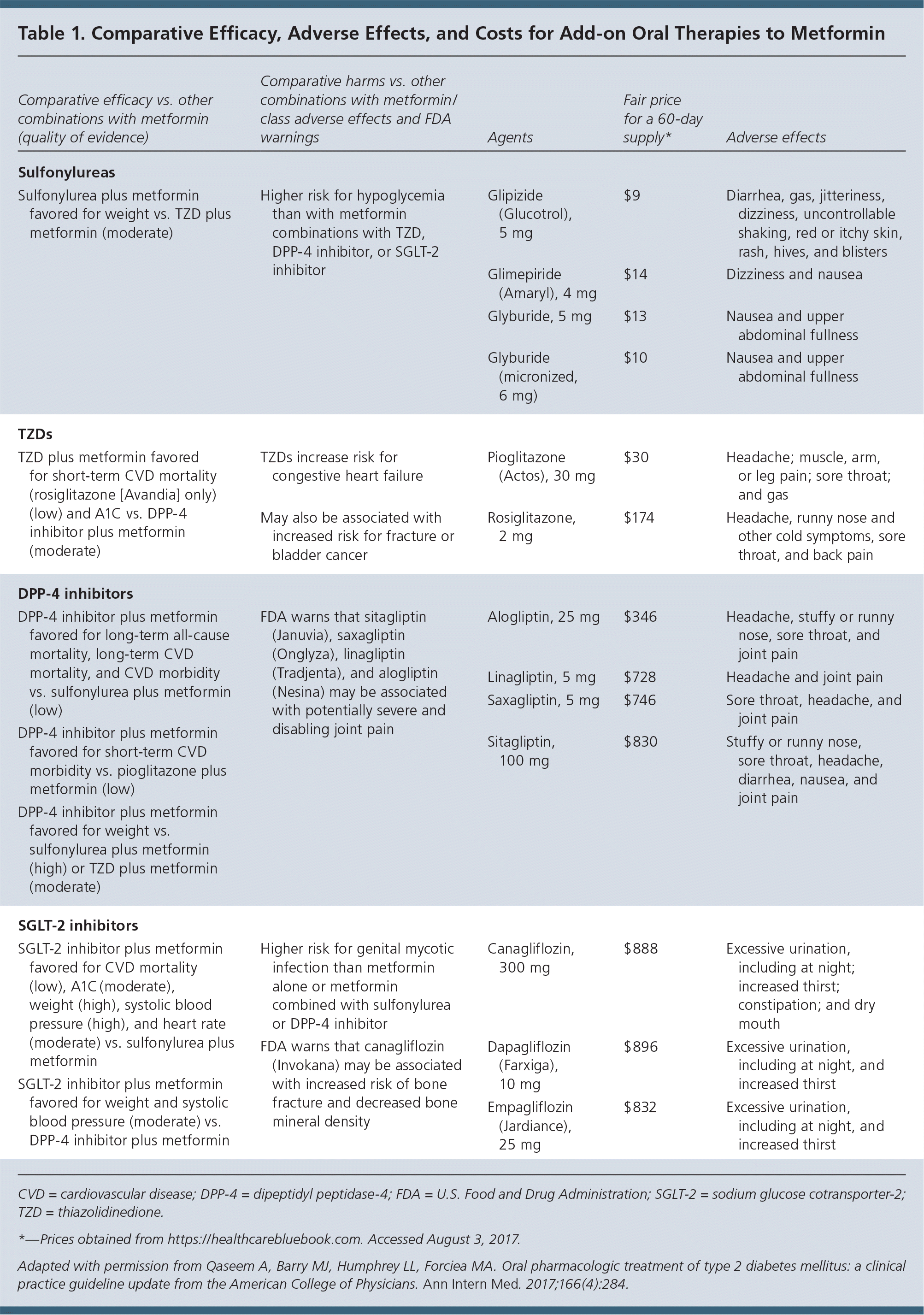
| Comparative efficacy vs. other combinations with metformin (quality of evidence) | Comparative harms vs. other combinations with metformin/class adverse effects and FDA warnings | Agents | Fair price for a 60-day supply* | Adverse effects |
|---|---|---|---|---|
| Sulfonylureas | ||||
| Sulfonylurea plus metformin favored for weight vs. TZD plus metformin (moderate) | Higher risk for hypoglycemia than with metformin combinations with TZD, DPP-4 inhibitor, or SGLT-2 inhibitor | Glipizide (Glucotrol), 5 mg | $9 | Diarrhea, gas, jitteriness, dizziness, uncontrollable shaking, red or itchy skin, rash, hives, and blisters |
| Glimepiride (Amaryl), 4 mg | $14 | Dizziness and nausea | ||
| Glyburide, 5 mg | $13 | Nausea and upper abdominal fullness | ||
| Glyburide (micronized, 6 mg) | $10 | Nausea and upper abdominal fullness | ||
| TZDs | ||||
| TZD plus metformin favored for short-term CVD mortality (rosiglitazone [Avandia] only) (low) and A1C vs. DPP-4 inhibitor plus metformin (moderate) | TZDs increase risk for congestive heart failure | Pioglitazone (Actos), 30 mg | $30 | Headache; muscle, arm, or leg pain; sore throat; and gas |
| May also be associated with increased risk for fracture or bladder cancer | Rosiglitazone, 2 mg | $174 | Headache, runny nose and other cold symptoms, sore throat, and back pain | |
| DPP-4 inhibitors | ||||
| DPP-4 inhibitor plus metformin favored for long-term all-cause mortality, long-term CVD mortality, and CVD morbidity vs. sulfonylurea plus metformin (low) | FDA warns that sitagliptin (Januvia), saxagliptin (Onglyza), linagliptin (Tradjenta), and alogliptin (Nesina) may be associated with potentially severe and disabling joint pain | Alogliptin, 25 mg | $346 | Headache, stuffy or runny nose, sore throat, and joint pain |
| DPP-4 inhibitor plus metformin favored for short-term CVD morbidity vs. pioglitazone plus metformin (low) | Linagliptin, 5 mg | $728 | Headache and joint pain | |
| DPP-4 inhibitor plus metformin favored for weight vs. sulfonylurea plus metformin (high) or TZD plus metformin (moderate) | Saxagliptin, 5 mg | $746 | Sore throat, headache, and joint pain | |
| Sitagliptin, 100 mg | $830 | Stuffy or runny nose, sore throat, headache, diarrhea, nausea, and joint pain | ||
| SGLT-2 inhibitors | ||||
| SGLT-2 inhibitor plus metformin favored for CVD mortality (low), A1C (moderate), weight (high), systolic blood pressure (high), and heart rate (moderate) vs. sulfonylurea plus metformin | Higher risk for genital mycotic infection than metformin alone or metformin combined with sulfonylurea or DPP-4 inhibitor | Canagliflozin, 300 mg | $888 | Excessive urination, including at night; increased thirst; constipation; and dry mouth |
| SGLT-2 inhibitor plus metformin favored for weight and systolic blood pressure (moderate) vs. DPP-4 inhibitor plus metformin | FDA warns that canagliflozin (Invokana) may be associated with increased risk of bone fracture and decreased bone mineral density | Dapagliflozin (Farxiga), 10 mg | $896 | Excessive urination, including at night, and increased thirst |
| Empagliflozin (Jardiance), 25 mg | $832 | Excessive urination, including at night, and increased thirst | ||
Sulfonylureas have the lowest cost but are associated with a greater risk of hypoglycemia and weight gain. SGLT-2 inhibitors are preferred to sulfonylureas and DPP-4 inhibitors based on their lesser effects on systolic blood pressure and weight, they are also preferred to sulfonylureas based on cardiovascular mortality, A1C, and heart rate. DPP-4 inhibitors are preferred over sulfonylureas with regard to long-term all-cause and cardiovascular mortality, as well as cardiovascular morbidity, and over sulfonylureas and thiazolidinediones with regard to weight gain.
It should be noted that combination therapy is associated with more adverse effects vs. treatment with metformin alone. Each drug has its own associated adverse effects, including increased risk of gastrointestinal issues with metformin, hypoglycemia with sulfonylureas, heart failure with thiazolidinediones, and genital mycotic infection with SGLT-2 inhibitors. Saxagliptin (Onglyza) and alogliptin (Nesina), both DPP-4 inhibitors, are associated with a greater risk of heart failure, especially in those who already have heart or kidney disease.
Guideline source: American College of Physicians
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Published source:Ann Intern Med. February 21, 2017;166(4):279–290
Endorsed by the AAFP, December 2016:https://www.aafp.org/patient-care/clinical-recommendations/all/type2-diabetes.html
LISA HAUK, AFP Senior Associate Editor
