
A more recent article on disorders of puberty is available.
Am Fam Physician. 2017;96(9):590-599
Patient information: See related handout on early and delayed puberty.
Author disclosure: No relevant financial affiliations.
Disorders of puberty can profoundly impact physical and psychosocial well-being. Precocious puberty is pubertal onset before eight years of age in girls and before nine years of age in boys. Patients with early isolated pubertal changes, prepubertal linear growth, and no worrisome neurologic symptoms typically have a benign pattern of development and should be monitored in the appropriate clinical context. Among patients with true precocious puberty, or full activation of the hypothalamic-pituitary-gonadal axis, most girls have an idiopathic etiology, whereas it is commonly due to identifiable pathology on imaging in boys. History and physical examination should be followed by measurements of serum follicle-stimulating hormone, luteinizing hormone, and testosterone (boys) or estradiol (girls); thyroid function testing; and bone age radiography. Brain magnetic resonance imaging should be performed in girls younger than six years, all boys with precocious puberty, and children with neurologic symptoms. Delayed puberty is the absence of breast development in girls by 13 years of age and absence of testicular growth to at least 4 mL in volume or 2.5 cm in length in boys by 14 years of age. Constitutional delay of growth and puberty is a common cause of delayed puberty; however, functional or persistent hypogonadism should be excluded. History and physical examination should be followed by measurements of serum follicle-stimulating hormone, luteinizing hormone, and testosterone (boys) or estradiol (girls); and bone age radiography. Abnormal growth velocity necessitates assessment of serum thyroid function, prolactin, and insulinlike growth factor I. Boys 14 years and older and girls 13 years and older may benefit from sex steroid treatment to jump-start puberty. Referral to a pediatric endocrinologist may be warranted after the initial evaluation.
Puberty is a developmental stage characterized by physical and psychosocial maturation. Abnormal pubertal timing can adversely affect a child's physical and psychosocial well-being and may be caused by a range of generally benign or pathologic etiologies. Physicians must identify which findings are suitable for surveillance over time and which suggest treatable underlying pathology.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Girls with signs of puberty before eight years of age and boys with signs of puberty before nine years of age should be evaluated for precocious puberty. | C | 5, 6 |
| Girls without breast development by 13 years of age should be evaluated for delayed puberty, and girls without menarche by 15 years of age should be evaluated for primary amenorrhea. | C | 5, 7, 25 |
| Boys who do not have testicular growth to at least 4 mL in volume or 2.5 cm in length by 14 years of age should be evaluated for delayed puberty. | C | 5, 7, 25 |
| In patients with precocious puberty, brain magnetic resonance imaging should be performed in girls younger than six years, all boys, and children with neurologic symptoms to evaluate for a central nervous system lesion. | C | 5, 6, 9 |
| Boys older than 14 years and girls older than 13 years with possible constitutional delay of growth and puberty may benefit from a short course of sex steroids to jump-start puberty. | C | 7, 25 |
Hormonal and Physical Changes of Normal Development
The physical changes of puberty are a result of gonadal sex hormone production, the start of which (gonadarche) indicates pubertal onset. Gonadarche is triggered by the pulsatile release of gonadotropin-releasing hormone, which activates the hypothalamic-pituitary-gonadal (HPG) axis.1–3 Adrenarche (i.e., adrenal androgen production leading to pubic and axillary hair, body odor, and mild acne) is a separate but usually concurrent process and does not in itself indicate true pubertal onset in boys or girls.4–7
In girls, increased ovarian estradiol secretion causes breast development at a mean age of 10 years (range: eight to 12 years). Menarche typically follows 2.5 years after the onset of breast development, at an average age of 12.5 years (range: nine to 15 years).1–5 In boys, testicular enlargement to at least 4 mL in volume or 2.5 cm in length is the first sign of true puberty and occurs at an average age of 11.5 years (range: 9.5 to 14 years).1–4,8–13 Physical changes are described using sexual maturity ratings (Table 11–5,9,14 and Table 21–5,9,14 ), such as Tanner stages, and are affected by body habitus and demographic factors.1–5,12,14
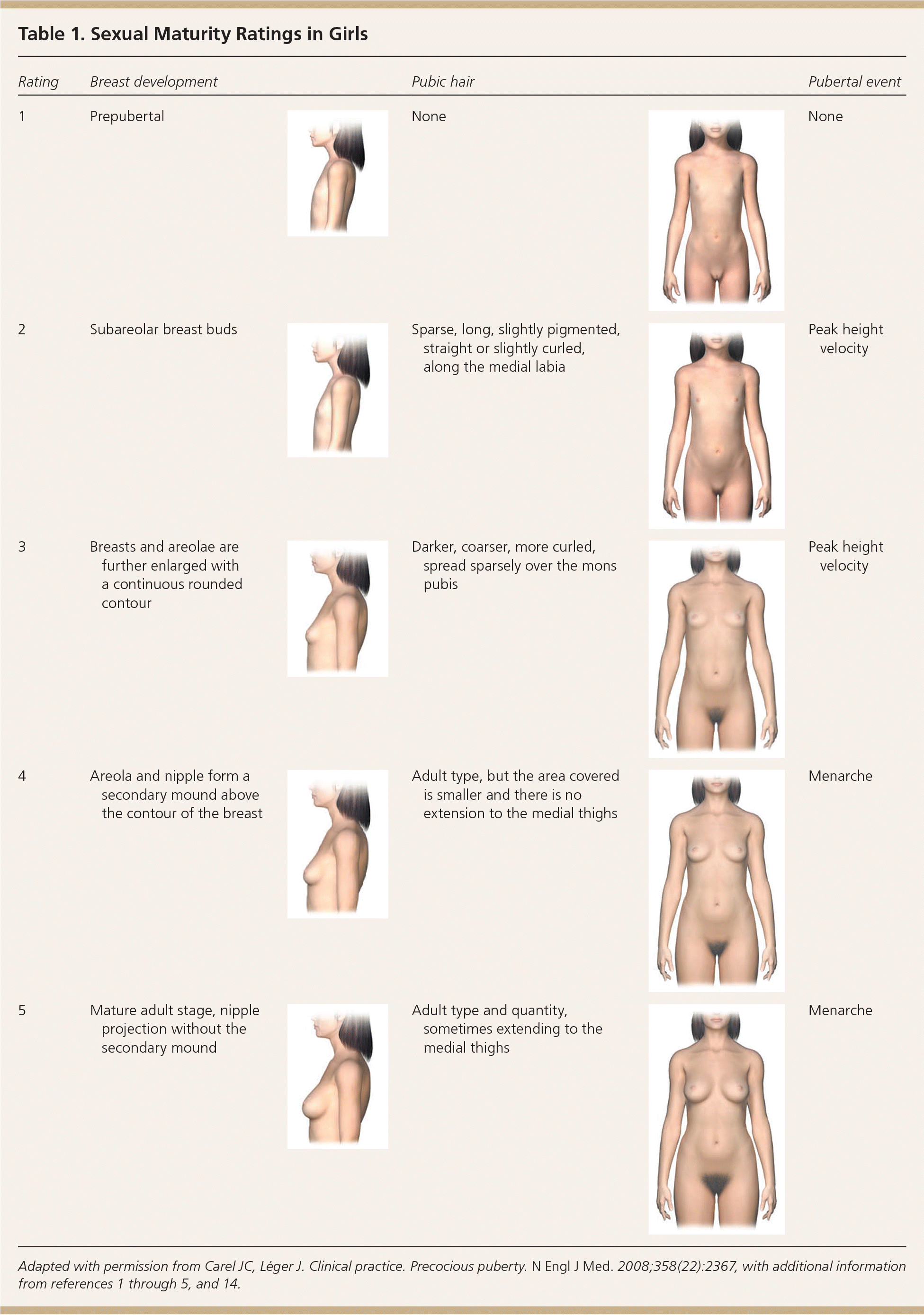
| Rating | Breast development | Pubic hair | Pubertal event | ||
|---|---|---|---|---|---|
| 1 | Prepubertal | None | None | ||
| 2 | Subareolar breast buds | Sparse, long, slightly pigmented, straight or slightly curled, along the medial labia | Peak height velocity | ||
| 3 | Breasts and areolae are further enlarged with a continuous rounded contour | Darker, coarser, more curled, spread sparsely over the mons pubis | Peak height velocity | ||
| 4 | Areola and nipple form a secondary mound above the contour of the breast | Adult type, but the area covered is smaller and there is no extension to the medial thighs | Menarche | ||
| 5 | Mature adult stage, nipple projection without the secondary mound | Adult type and quantity, sometimes extending to the medial thighs | Menarche | ||
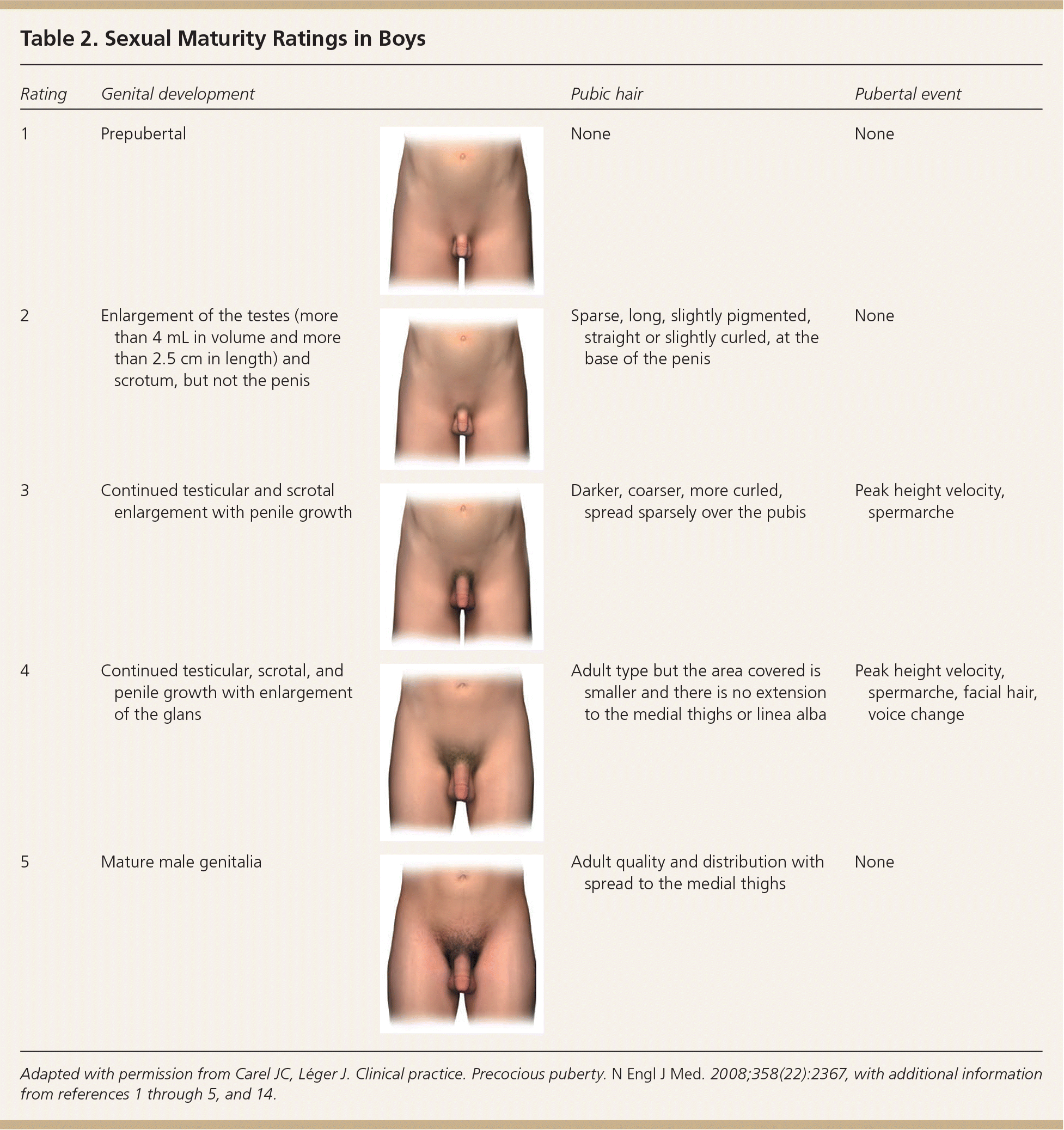
| Rating | Genital development | Pubic hair | Pubertal event | |
|---|---|---|---|---|
| 1 | Prepubertal | None | None | |
| 2 | Enlargement of the testes (more than 4 mL in volume and more than 2.5 cm in length) and scrotum, but not the penis | Sparse, long, slightly pigmented, straight or slightly curled, at the base of the penis | None | |
| 3 | Continued testicular and scrotal enlargement with penile growth | Darker, coarser, more curled, spread sparsely over the pubis | Peak height velocity, spermarche | |
| 4 | Continued testicular, scrotal, and penile growth with enlargement of the glans | Adult type but the area covered is smaller and there is no extension to the medial thighs or linea alba | Peak height velocity, spermarche, facial hair, voice change | |
| 5 | Mature male genitalia | Adult quality and distribution with spread to the medial thighs | None | |
Linear growth velocity is about 5 cm per year from four years of age to puberty with a nadir before the pubertal growth spurt. Girls achieve peak height velocity during sexual maturity ratings 2 and 3 (mean: 8.3 cm per year, age 11 or 12 years) and boys during sexual maturity ratings 3 and 4 (mean: 9.5 cm per year, age 13 or 14 years). On average, girls complete linear growth at 15 years of age and boys at 17 years of age. After menarche, girls grow an average of 7 cm.1–4,14–18
When to Suspect a Disorder of Puberty
PRECOCIOUS PUBERTY
Precocious puberty is diagnosed when secondary sexual characteristics are identified in girls younger than eight years and boys younger than nine years.5,6 Data suggest a trend toward early pubertal development. Approximately 20% of black girls and 5% to 10% of white girls seven to eight years of age in the United States have glandular breast development, particularly if obese.19–23 Eight years of age can be considered a reasonable cutoff for evaluation in girls.5,6 Because of more frequent pathology in boys with precocious puberty than girls, all pubertal boys younger than nine years should be fully evaluated.5,6,24
DELAYED PUBERTY
Puberty is considered delayed when there are no signs of breast development by 13 years of age in girls or testicular enlargement by 14 years of age in boys.5,7,25 Clinicians should suspect pubertal delay if there is halting or regression of pubertal development. In girls with initial pubertal changes, absence of menarche by 15 years of age is also concerning.
Evaluation of a Suspected Disorder of Puberty
HISTORY
The clinician should inquire about the onset and progression of body odor, acne, breast or testicular development, and pubic and axillary hair. Current or previous therapies, including chemotherapy, radiation therapy, or exogenous sex steroids, may indicate the underlying etiology. Neurologic symptoms may reveal intracranial pathology. For delayed puberty, a history suggestive of underlying chronic disease (e.g., fatigue, pain, abnormal stools), nutrition and exercise patterns, poor psychosocial functioning, cryptorchidism, anosmia [i.e., in Kallmann syndrome]) is important.
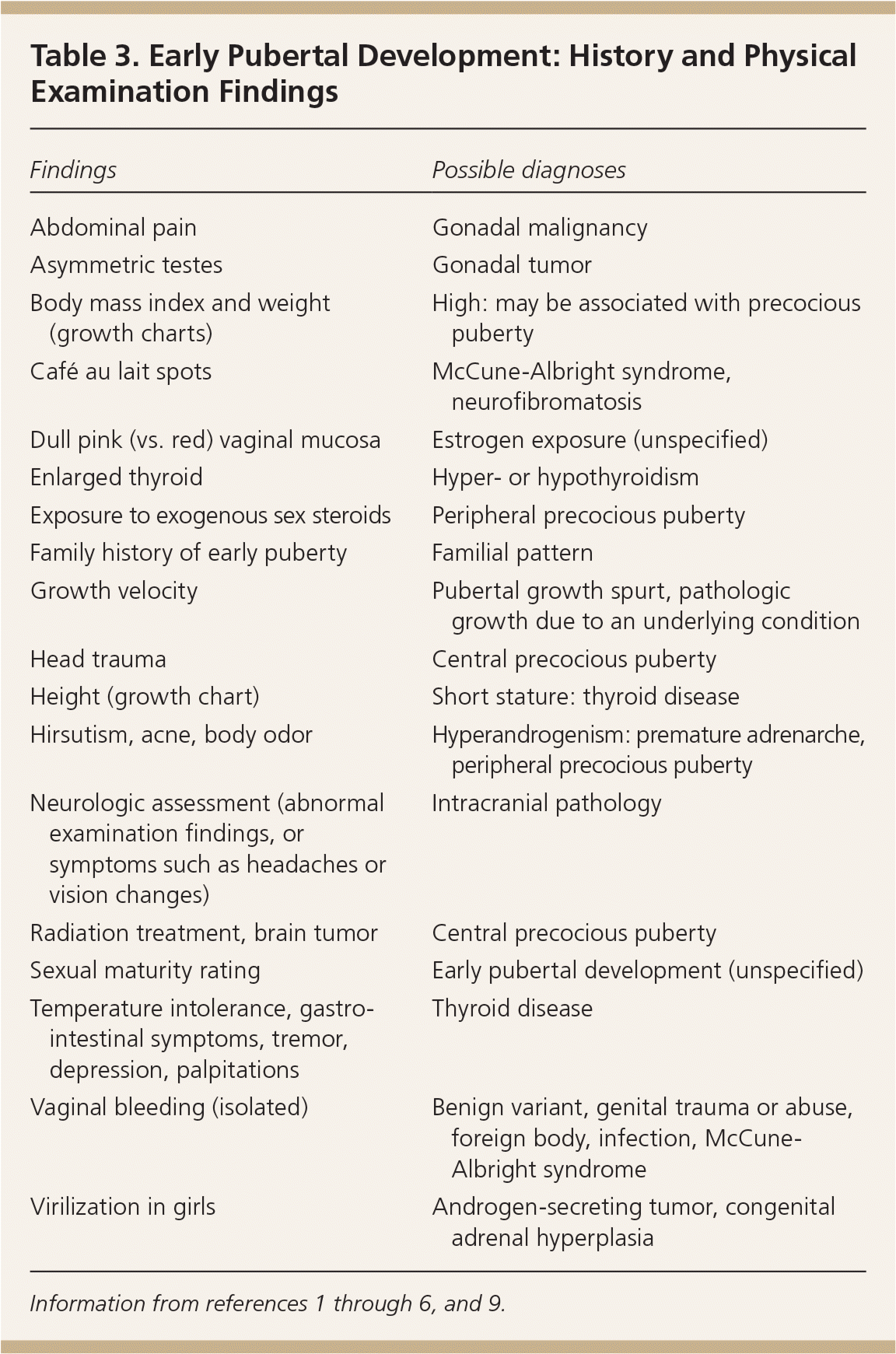
| Findings | Possible diagnoses |
|---|---|
| Abdominal pain | Gonadal malignancy |
| Asymmetric testes | Gonadal tumor |
| Body mass index and weight (growth charts) | High: may be associated with precocious puberty |
| Café au lait spots | McCune-Albright syndrome, neurofibromatosis |
| Dull pink (vs. red) vaginal mucosa | Estrogen exposure (unspecified) |
| Enlarged thyroid | Hyper- or hypothyroidism |
| Exposure to exogenous sex steroids | Peripheral precocious puberty |
| Family history of early puberty | Familial pattern |
| Growth velocity | Pubertal growth spurt, pathologic growth due to an underlying condition |
| Head trauma | Central precocious puberty |
| Height (growth chart) | Short stature: thyroid disease |
| Hirsutism, acne, body odor | Hyperandrogenism: premature adrenarche, peripheral precocious puberty |
| Neurologic assessment (abnormal examination findings, or symptoms such as headaches or vision changes) | Intracranial pathology |
| Radiation treatment, brain tumor | Central precocious puberty |
| Sexual maturity rating | Early pubertal development (unspecified) |
| Temperature intolerance, gastrointestinal symptoms, tremor, depression, palpitations | Thyroid disease |
| Vaginal bleeding (isolated) | Benign variant, genital trauma or abuse, foreign body, infection, McCune-Albright syndrome |
| Virilization in girls | Androgen-secreting tumor, congenital adrenal hyperplasia |

| Findings | Possible diagnoses |
|---|---|
| Abdominal pain | Gastrointestinal disease |
| Anosmia | Kallmann syndrome |
| Asymmetric testes | Oophoritis or orchitis |
| Body mass index and weight (on growth charts) | Low: eating disorder, caloric insufficiency, gastrointestinal or other systemic disease |
| Chemotherapy, radiation treatment, brain tumor | Hypogonadism |
| Cryptorchidism or orchidopexy | Hypogonadism |
| Dysmorphic features (webbed neck, short stature, low hairline) | Turner syndrome |
| Enlarged thyroid | Hypothyroidism |
| Family history of late puberty | Constitutional delay of growth and puberty |
| Galactorrhea | Hyperprolactinemia |
| Growth velocity | Peripubertal growth slowing, pathologic growth due to underlying condition |
| Height (growth chart) | Short stature: Turner syndrome, constitutional delay of growth and puberty |
| Tall stature: Klinefelter syndrome | |
| Joint pain | Inflammatory disorder |
| Neurologic assessment (abnormal examination findings or symptoms such as headaches, vision changes) | Intracranial pathology |
| Red (vs. dull pink) or thin vaginal mucosa | Lack of estrogen exposure (hypogonadism) |
| Sexual maturity rating | Delayed pubertal development (unspecified) |
| Small, firm testes | Klinefelter syndrome |
| Temperature intolerance, gastrointestinal symptoms, tremor, depression, palpitations | Thyroid disease |
| Trauma (head) | Hypogonadism |
| Vasomotor symptoms in girls | Ovarian insufficiency |
| Weight loss, stress, excessive exercise, inadequate nutrition, fatigue | Eating disorder, caloric insufficiency |
PHYSICAL EXAMINATION
Height, weight, and body mass index should be plotted on growth curves, and the height velocity should be calculated.3,23 Target height (midparental height) can be determined using the following equation: [mother's height + father's height + 13 cm in boys or − 13 cm in girls] ÷ 2.18,26 A target height differing from the projected height, as established by extending the growth curve to adulthood or bone age radiography, by approximately more than 10 cm may suggest a pathologic condition.26 Because of the effects of sex steroids on epiphyseal maturation, patients with precocious puberty may present with relatively tall stature (leading to shorter adult height), and those with delayed puberty may present with short stature.26
The patient's sexual maturity rating should be noted, as well as the amounts of acne and axillary and facial hair. In boys, determining the location, consistency, and size of the testes can evaluate for cryptorchidism, malignancy, or Klinefelter syndrome (firm testes), and help determine pubertal staging. In girls, dull pink vaginal mucosa suggests estrogen exposure; virilization (e.g., clitoromegaly) should be excluded.4–7,9,27
Early Pubertal Development
eTable A includes the differential diagnosis of isolated pubertal changes and true precocious puberty.
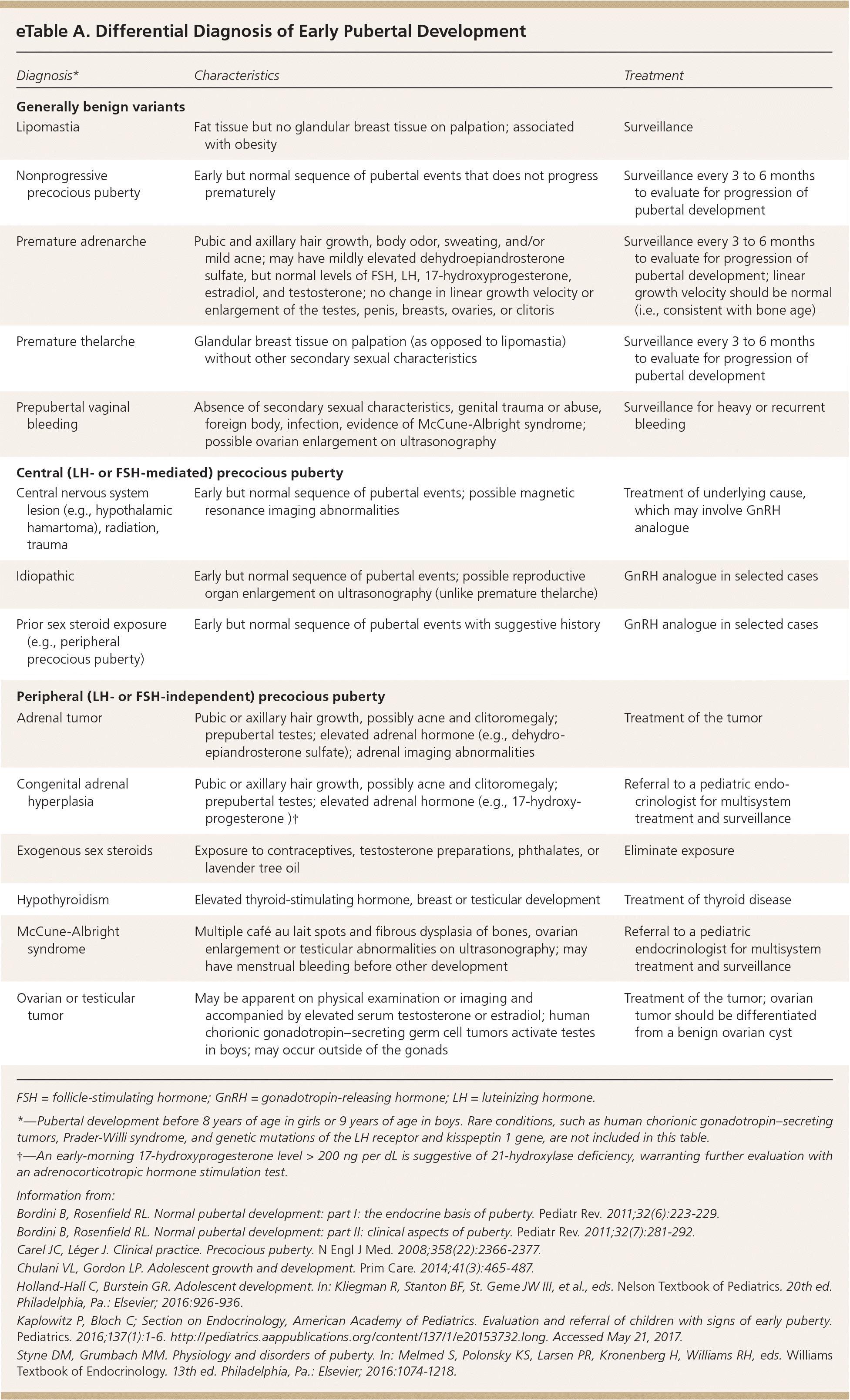
| Diagnosis* | Characteristics | Treatment |
|---|---|---|
| Generally benign variants | ||
| Lipomastia | Fat tissue but no glandular breast tissue on palpation; associated with obesity | Surveillance |
| Nonprogressive precocious puberty | Early but normal sequence of pubertal events that does not progress prematurely | Surveillance every 3 to 6 months to evaluate for progression of pubertal development |
| Premature adrenarche | Pubic and axillary hair growth, body odor, sweating, and/or mild acne; may have mildly elevated dehydroepiandrosterone sulfate, but normal levels of FSH, LH, 17-hydroxyprogesterone, estradiol, and testosterone; no change in linear growth velocity or enlargement of the testes, penis, breasts, ovaries, or clitoris | Surveillance every 3 to 6 months to evaluate for progression of pubertal development; linear growth velocity should be normal (i.e., consistent with bone age) |
| Premature thelarche | Glandular breast tissue on palpation (as opposed to lipomastia) without other secondary sexual characteristics | Surveillance every 3 to 6 months to evaluate for progression of pubertal development |
| Prepubertal vaginal bleeding | Absence of secondary sexual characteristics, genital trauma or abuse, foreign body, infection, evidence of McCune-Albright syndrome; possible ovarian enlargement on ultrasonography | Surveillance for heavy or recurrent bleeding |
| Central (LH‐ or FSH ‐mediated) precocious puberty | ||
| Central nervous system lesion (e.g., hypothalamic hamartoma), radiation, trauma | Early but normal sequence of pubertal events; possible magnetic resonance imaging abnormalities | Treatment of underlying cause, which may involve GnRH analogue |
| Idiopathic | Early but normal sequence of pubertal events; possible reproductive organ enlargement on ultrasonography (unlike premature thelarche) | GnRH analogue in selected cases |
| Prior sex steroid exposure (e.g., peripheral precocious puberty) | Early but normal sequence of pubertal events with suggestive history | GnRH analogue in selected cases |
| Peripheral (LH‐ or FSH‐independent) precocious puberty | ||
| Adrenal tumor | Pubic or axillary hair growth, possibly acne and clitoromegaly; prepubertal testes; elevated adrenal hormone (e.g., dehydro-epiandrosterone sulfate); adrenal imaging abnormalities | Treatment of the tumor |
| Congenital adrenal hyperplasia | Pubic or axillary hair growth, possibly acne and clitoromegaly; prepubertal testes; elevated adrenal hormone (e.g., 17-hydroxy-progesterone)† | Referral to a pediatric endocrinologist for multisystem treatment and surveillance |
| Exogenous sex steroids | Exposure to contraceptives, testosterone preparations, phthalates, or lavender tree oil | Eliminate exposure |
| Hypothyroidism | Elevated thyroid-stimulating hormone, breast or testicular development | Treatment of thyroid disease |
| McCune-Albright syndrome | Multiple café au lait spots and fibrous dysplasia of bones, ovarian enlargement or testicular abnormalities on ultrasonography; may have menstrual bleeding before other development | Referral to a pediatric endocrinologist for multisystem treatment and surveillance |
| Ovarian or testicular tumor | May be apparent on physical examination or imaging and accompanied by elevated serum testosterone or estradiol; human chorionic gonadotropin–secreting germ cell tumors activate testes in boys; may occur outside of the gonads | Treatment of the tumor; ovarian tumor should be differentiated from a benign ovarian cyst |
ISOLATED PUBERTAL CHANGES
Premature thelarche, defined by isolated glandular breast tissue on palpation, should be differentiated from lipomastia (isolated fatty breast tissue), which is common in obese children.21 To differentiate these conditions, clinicians may examine the patient in the supine position, thereby making the breasts less prominent, to determine presence or absence of glandular tissue under the areolae. Isolated prepubertal vaginal bleeding not caused by trauma, abuse, a foreign body, infection, or an exceedingly rare tumor is usually benign.6,28
Premature adrenarche, driven by adrenal androgens rather than activation of the HPG axis, leads to slowly progressive appearance of pubic and axillary hair, body odor, sweating, and/or mild acne without change in linear growth velocity or enlargement of the testes, penis, breasts, ovaries, or clitoris. Dehydroepiandrosterone sulfate may be at a pubertal level (i.e., slightly elevated for the patient's chronologic age), whereas estradiol, testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) remain at prepubertal levels.5,6,9 Less than 5% of patients have an elevated 17-hydroxyprogesterone level, suggesting mild nonclassic congenital adrenal hyperplasia, which does not usually require treatment. Thus, laboratory evaluation for such isolated findings may be delayed.6 Deferring laboratory tests also applies in cases of fine, sparse pubic hair growth that sometimes occurs in infancy.6
Patients with early isolated pubertal changes, prepubertal linear growth, and no worrisome neurologic symptoms typically have a benign pattern of development, necessitating only surveillance over three to six months to evaluate for progression.3–6,9,29 Laboratory or bone age assessment may be deferred initially. Notably, bone age advancement by two standard deviations has low predictive value in differentiating benign pubertal variants from concerning causes of precocious puberty.6,30
Gynecomastia, or estrogen-mediated glandular breast tissue, is common in pubertal boys. Evaluation for chronic disease; hyperprolactinemia; testicular or adrenal neoplasm; use of prescription, recreational, or performance-enhancing drugs; or hypogonadism (e.g., Klinefelter syndrome) should be initiated if symptoms persist for 18 to 24 months or the patient has no pubertal changes.31
CENTRAL AND PERIPHERAL PRECOCIOUS PUBERTY
Precocious puberty can be characterized by the pathologic location. In central precocious puberty, the HPG axis is activated, resulting in early but normal development, symmetric progression of secondary sexual characteristics, and increasing growth velocity.6,9,32 Central precocious puberty is approximately 10-fold more common in girls than in boys.33 Although usually idiopathic in girls, it can be incited by head trauma, neoplasm, radiation, or genetic conditions.5,6,9 Pathologic causes of central precocious puberty are more common in boys.5,6,9
Peripheral precocious puberty occurs when hormonal influences originating outside of the HPG axis produce incomplete, atypically sequenced or rapid pubertal progression.5,6,9 Quickly progressing or significant hyperandrogenic findings may warrant workup for congenital adrenal hyperplasia or an androgen-secreting tumor. Elevated estradiol levels in the setting of low LH may suggest an estrogen-secreting tumor.6 Hypothyroidism and exogenous steroid use should be excluded. Multiple café au lait spots and fibrous dysplasia of bones are concerning for McCune-Albright syndrome or neurofibromatosis.5,6,9
The initial workup should include measurement of serum FSH, LH, and testosterone in boys or estradiol in girls; thyroid function testing; and bone age radiography (eTable B, Figure 15,6,9). In cases of hyperandrogenic findings, measuring serum dehydroepiandrosterone sulfate and 17-hydroxyprogesterone is indicated. An LH level of more than 0.3 mIU per mL (0.3 IU per L) is the most reliable laboratory finding for central precocious puberty; however, in patients with lower values and high clinical suspicion, a gonadotropin-releasing hormone analogue stimulation test may be warranted.6,34 In cases of diagnostic uncertainty, pelvic ultrasonography can evaluate for increased uterine and ovarian volume expected for age, which may indicate central precocious puberty or a tumor.6
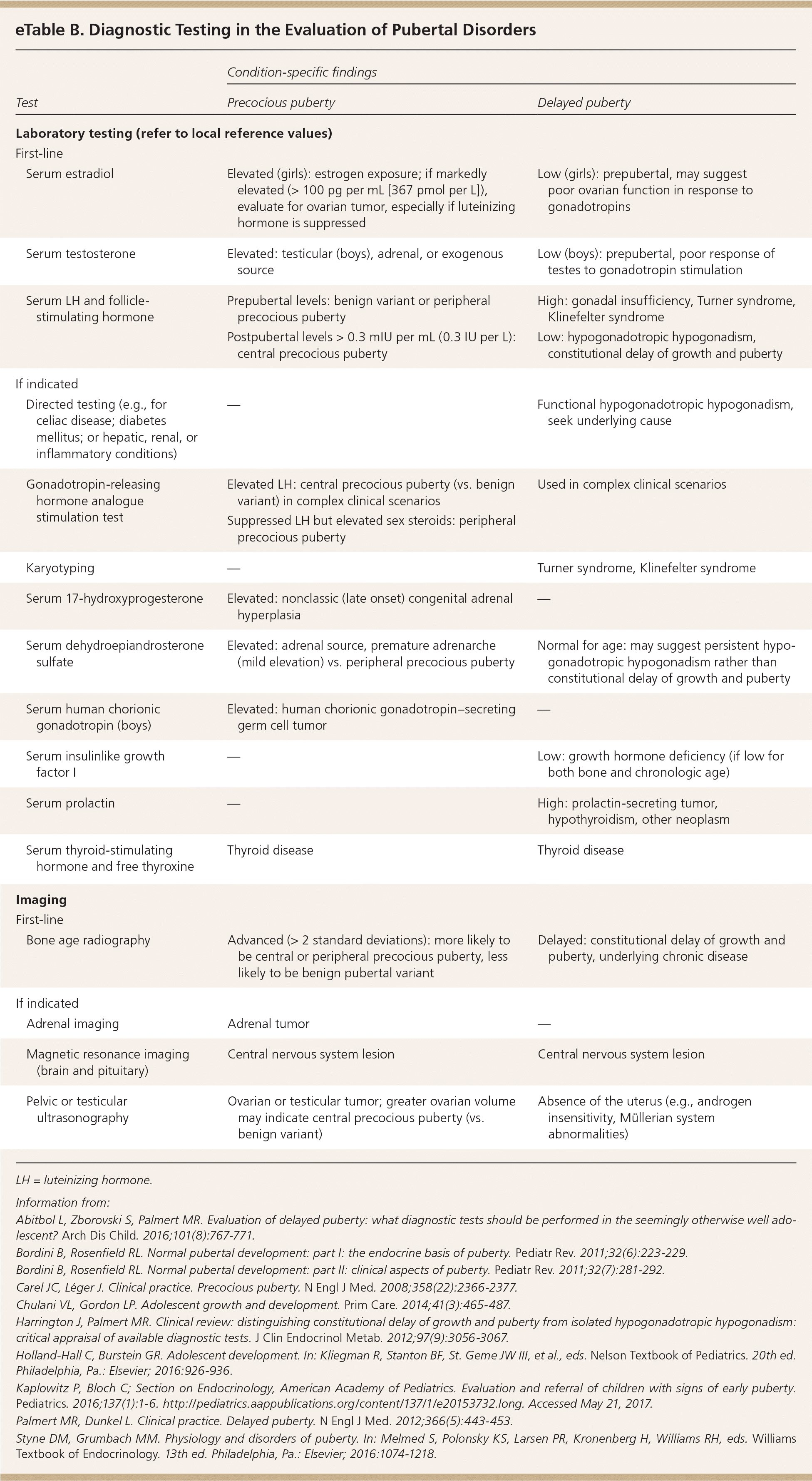
| Test | Condition-specific findings | ||
|---|---|---|---|
| Precocious puberty | Delayed puberty | ||
| Laboratory testing (refer to local reference values) | |||
| First-line | |||
| Serum estradiol | Elevated (girls): estrogen exposure; if markedly elevated (> 100 pg per mL [367 pmol per L]), evaluate for ovarian tumor, especially if luteinizing hormone is suppressed | Low (girls): prepubertal, may suggest poor ovarian function in response to gonadotropins | |
| Serum testosterone | Elevated: testicular (boys), adrenal, or exogenous source | Low (boys): prepubertal, poor response of testes to gonadotropin stimulation | |
| Serum LH and follicle-stimulating hormone | Prepubertal levels: benign variant or peripheral precocious puberty | High: gonadal insufficiency, Turner syndrome, Klinefelter syndrome | |
| Postpubertal levels > 0.3 mIU per mL (0.3 IU per L): central precocious puberty | Low: hypogonadotropic hypogonadism, constitutional delay of growth and puberty | ||
| If indicated | |||
| Directed testing (e.g., for celiac disease; diabetes mellitus; or hepatic, renal, or inflammatory conditions) | — | Functional hypogonadotropic hypogonadism, seek underlying cause | |
| Gonadotropin-releasing hormone analogue stimulation test | Elevated LH: central precocious puberty (vs. benign variant) in complex clinical scenarios | Used in complex clinical scenarios | |
| Suppressed LH but elevated sex steroids: peripheral precocious puberty | |||
| Karyotyping | — | Turner syndrome, Klinefelter syndrome | |
| Serum 17-hydroxyprogesterone | Elevated: nonclassic (late onset) congenital adrenal hyperplasia | — | |
| Serum dehydroepiandrosterone sulfate | Elevated: adrenal source, premature adrenarche (mild elevation) vs. peripheral precocious puberty | Normal for age: may suggest persistent hypogonadotropic hypogonadism rather than constitutional delay of growth and puberty | |
| Serum human chorionic gonadotropin (boys) | Elevated: human chorionic gonadotropin–secreting germ cell tumor | — | |
| Serum insulinlike growth factor I | — | Low: growth hormone deficiency (if low for both bone and chronologic age) | |
| Serum prolactin | — | High: prolactin-secreting tumor, hypothyroidism, other neoplasm | |
| Serum thyroid-stimulating hormone and free thyroxine | Thyroid disease | Thyroid disease | |
| Imaging | |||
| First-line | |||
| Bone age radiography | Advanced (> 2 standard deviations): more likely to be central or peripheral precocious puberty, less likely to be benign pubertal variant | Delayed: constitutional delay of growth and puberty, underlying chronic disease | |
| If indicated | |||
| Adrenal imaging | Adrenal tumor | — | |
| Magnetic resonance imaging (brain and pituitary) | Central nervous system lesion | Central nervous system lesion | |
| Pelvic or testicular ultrasonography | Ovarian or testicular tumor; greater ovarian volume may indicate central precocious puberty (vs. benign variant) | Absence of the uterus (e.g., androgen insensitivity, Müllerian system abnormalities) | |
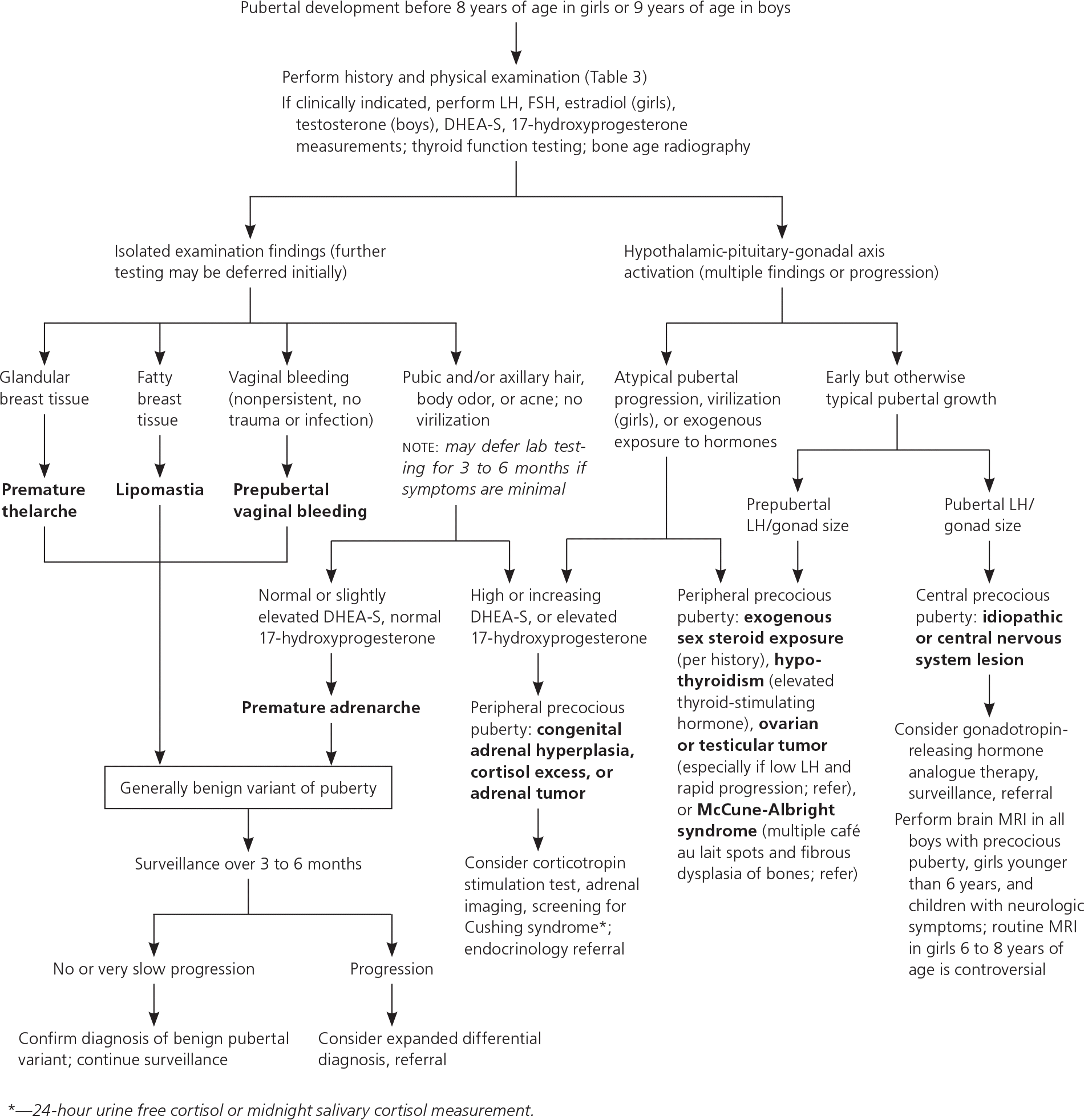
The appropriate timing for neuroimaging to identify central nervous system lesions (e.g., hypothalamic hamartoma, malignancy) in children with precocious puberty is controversial. Girls younger than six years, all boys with precocious puberty, and children with neurologic symptoms such as headache, vision changes, or seizures should be screened with magnetic resonance imaging.5,6,9 Some experts discourage routine neuro-imaging for asymptomatic girls six to eight years of age because pathology requiring treatment is exceedingly rare. With shared decision making, parents can weigh the risks of sedation, intravenous contrast media, and follow-up imaging (leading to anxiety and high cost) against the low likelihood that imaging will show a new central nervous system malignancy (at most 1%).5,6,35
If started early in the course of central precocious puberty, gonadotropin-releasing hormone analogues (e.g., leuprolide [Lupron]) appear to safely prevent premature fusion of growth plates, thereby preserving height potential.36 Because of high annual costs, treatment may be most appropriate if bone age suggests impending short stature or if the patient exhibits aggression (boys) or profound emotionality in response to menses (girls).10,37
Delayed Puberty
Delayed puberty is the absence of breast development by 13 years of age in girls or the absence of testicular growth to at least 4 mL in volume or 2.5 cm in length by 14 years of age in boys.7–9,25,38 Constitutional delay of growth and puberty is the most common cause of delayed puberty in boys (60%) and girls (30%).39,40 It represents an extreme of the normal spectrum of pubertal timing and is a diagnosis of exclusion.39,40 For more than 75% of patients with constitutional delay of growth and puberty, family history may reveal parental pubertal delay.41,42
Other etiologies of delayed puberty are categorized based on gonadotropin levels. In hypergonadotropic hypogonadism, gonadal insufficiency delays puberty and results in elevated levels of FSH and LH. Conditions causing hypergonadotropic hypogonadism can be congenital or acquired and are collectively more common in girls (26%) than in boys (7%) with delayed puberty.7,39
Hypogonadotropic hypogonadism is characterized by low levels of FSH and LH and further classified by the pathology. Functional hypogonadotropic hypogonadism is caused by chronic disease, stress, or inadequate nutrition, and the condition may be transient or reversed. Persistent hypogonadotropic hypogonadism is caused by a congenital abnormality in the HPG axis or an acquired etiology such as a central nervous system tumor, trauma, surgery, or radiation.7,43 Patients with persistent hypogonadotropic hypogonadism require treatment to induce puberty, maintain normal adult levels of sex steroids, and optimize fertility.44 eTable C includes the differential diagnosis of delayed or absent puberty.
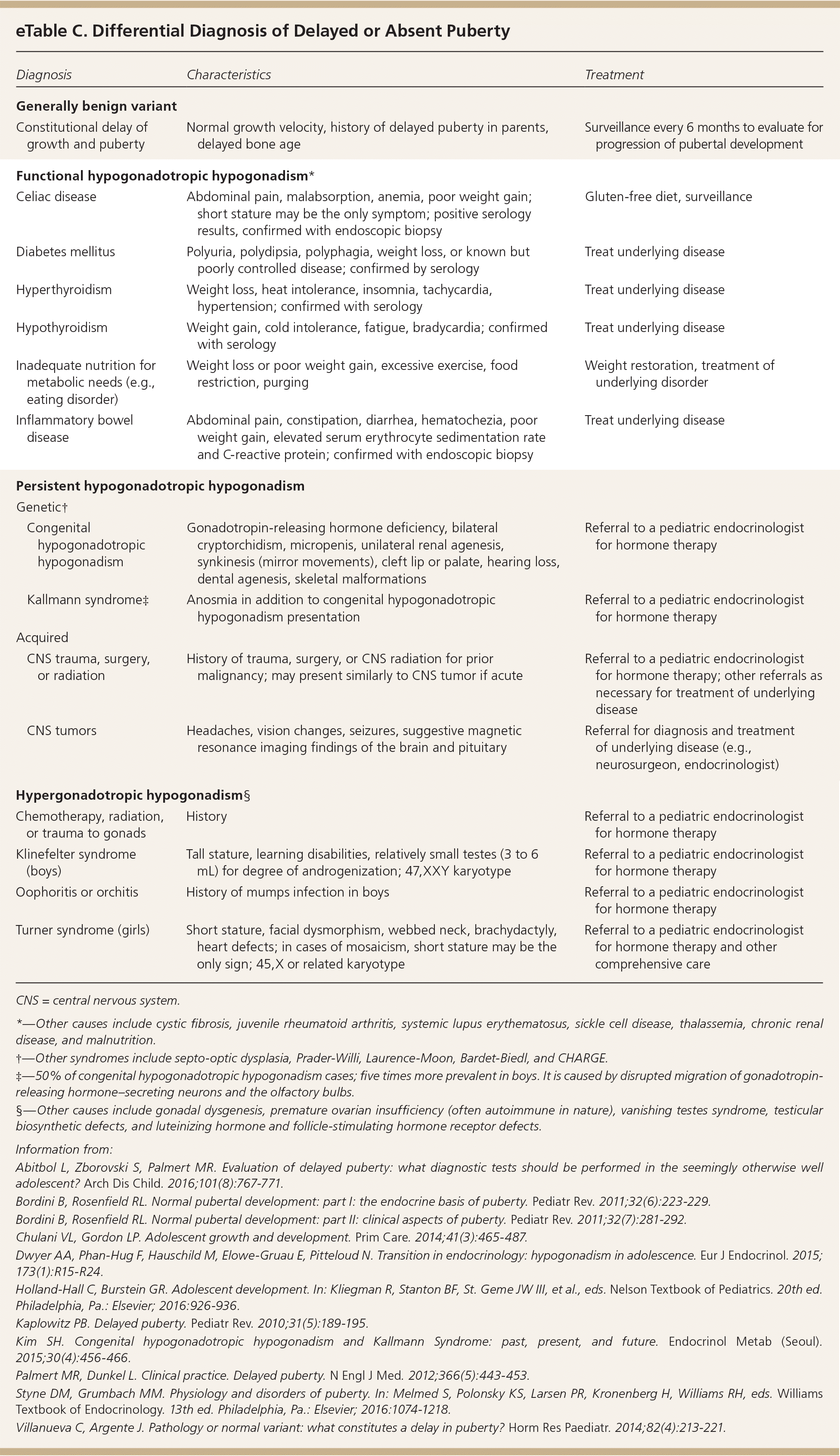
| Diagnosis | Characteristics | Treatment | |
|---|---|---|---|
| Generally benign variant | |||
| Constitutional delay of growth and puberty | Normal growth velocity, history of delayed puberty in parents, delayed bone age | Surveillance every 6 months to evaluate for progression of pubertal development | |
| Functional hypogonadotropic hypogonadism* | |||
| Celiac disease | Abdominal pain, malabsorption, anemia, poor weight gain; short stature may be the only symptom; positive serology results, confirmed with endoscopic biopsy | Gluten-free diet, surveillance | |
| Diabetes mellitus | Polyuria, polydipsia, polyphagia, weight loss, or known but poorly controlled disease; confirmed by serology | Treat underlying disease | |
| Hyperthyroidism | Weight loss, heat intolerance, insomnia, tachycardia, hypertension; confirmed with serology | Treat underlying disease | |
| Hypothyroidism | Weight gain, cold intolerance, fatigue, bradycardia; confirmed with serology | Treat underlying disease | |
| Inadequate nutrition for metabolic needs (e.g., eating disorder) | Weight loss or poor weight gain, excessive exercise, food restriction, purging | Weight restoration, treatment of underlying disorder | |
| Inflammatory bowel disease | Abdominal pain, constipation, diarrhea, hematochezia, poor weight gain, elevated serum erythrocyte sedimentation rate and C-reactive protein; confirmed with endoscopic biopsy | Treat underlying disease | |
| Persistent hypogonadotropic hypogonadism | |||
| Genetic† | |||
| Congenital hypogonadotropic hypogonadism | Gonadotropin-releasing hormone deficiency, bilateral cryptorchidism, micropenis, unilateral renal agenesis, synkinesis (mirror movements), cleft lip or palate, hearing loss, dental agenesis, skeletal malformations | Referral to a pediatric endocrinologist for hormone therapy | |
| Kallmann syndrome‡ | Anosmia in addition to congenital hypogonadotropic hypogonadism presentation | Referral to a pediatric endocrinologist for hormone therapy | |
| Acquired | |||
| CNS trauma, surgery, or radiation | History of trauma, surgery, or CNS radiation for prior malignancy; may present similarly to CNS tumor if acute | Referral to a pediatric endocrinologist for hormone therapy; other referrals as necessary for treatment of underlying disease | |
| CNS tumors | Headaches, vision changes, seizures, suggestive magnetic resonance imaging findings of the brain and pituitary | Referral for diagnosis and treatment of underlying disease (e.g., neurosurgeon, endocrinologist) | |
| Hypergonadotropic hypogonadism§ | |||
| Chemotherapy, radiation, or trauma to gonads | History | Referral to a pediatric endocrinologist for hormone therapy | |
| Klinefelter syndrome (boys) | Tall stature, learning disabilities, relatively small testes (3 to 6 mL) for degree of androgenization; 47,XXY karyotype | Referral to a pediatric endocrinologist for hormone therapy | |
| Oophoritis or orchitis | History of mumps infection in boys | Referral to a pediatric endocrinologist for hormone therapy | |
| Turner syndrome (girls) | Short stature, facial dysmorphism, webbed neck, brachydactyly, heart defects; in cases of mosaicism, short stature may be the only sign; 45,X or related karyotype | Referral to a pediatric endocrinologist for hormone therapy and other comprehensive care | |
Initial workup should include measurements of serum FSH, LH, testosterone in boys or estradiol in girls, and bone age radiography (eTable B, Figure 27,8,25,44,45). If abnormal growth velocity is a concern, serum thyroid function, prolactin, and insulinlike growth factor I should be assessed.7 Constitutional delay of growth and puberty can be difficult to distinguish from persistent hypogonadotropic hypogonadism; the latter may be diagnosed at 18 years of age if there is inadequate response to jump-start therapy (which is defined later in this section), and sex steroid replacement is still required.7,45
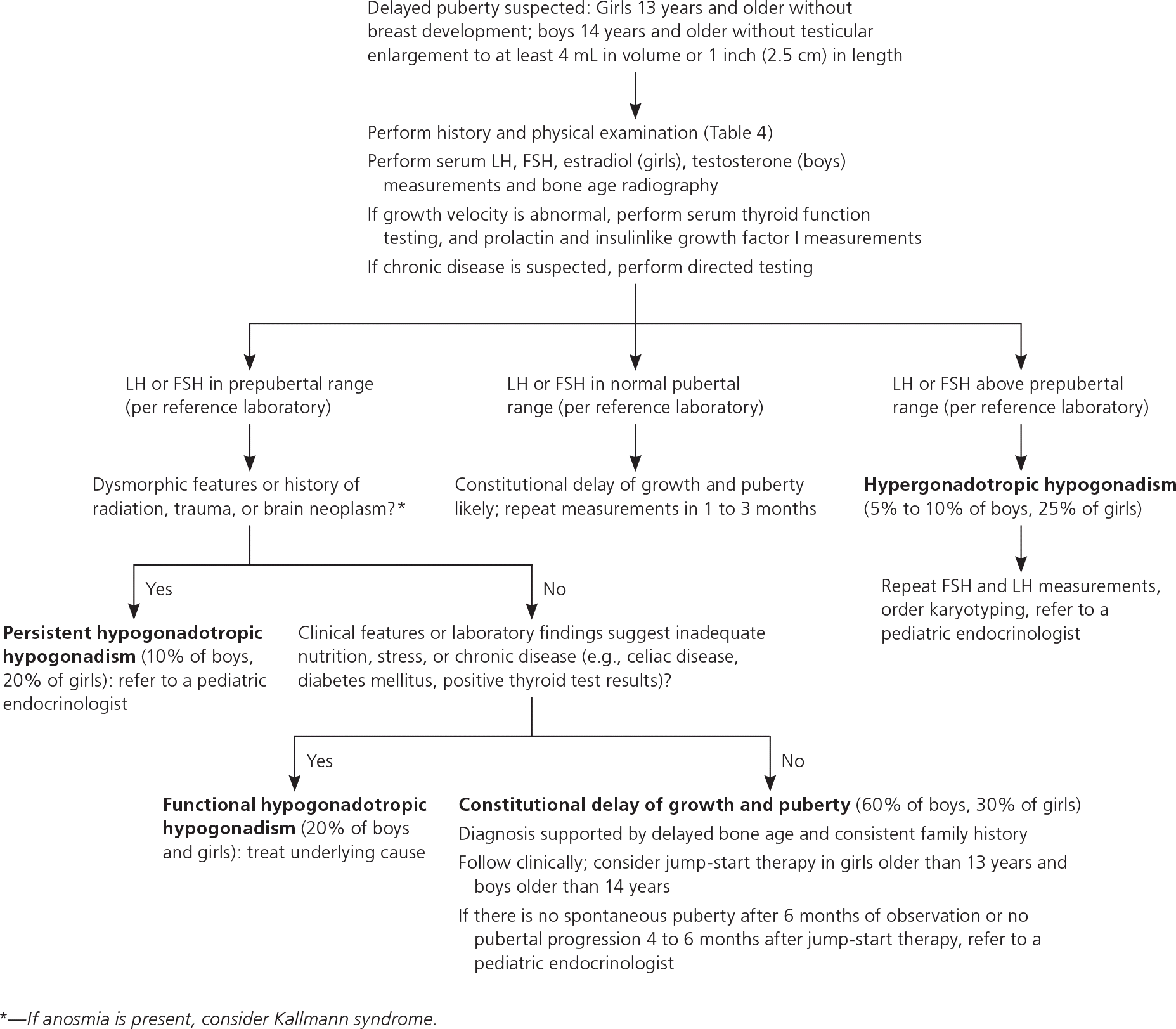
Delayed puberty can cause significant psychological distress and low self-esteem.46,47 Girls older than 13 years and boys older than 14 years with possible constitutional delay of growth and puberty or gonadotropin-releasing hormone deficiency may be offered jump-start therapy to induce puberty.5,7,8,25,45 For example, treating boys with testosterone cypionate or enanthate (e.g., 50 to 100 mg intramuscularly per month) and girls with overnight transdermal estradiol (e.g., 6.2 mcg, one-fourth of the 25-mcg 24-hour patch) for three to six months may accelerate attainment of final adult height and generally does not lead to premature epiphysis closure.7,25 If pubertal progression does not occur within four to six months after completing therapy, further evaluation for persistent hypogonadotropic hypogonadism and long-term hormone therapy should be initiated.5,7 Indications for referral to a pediatric endocrinologist are listed in eTable D.

| Concern for early puberty* | |
| Any pubertal changes before 6 years of age in girls and 9 years of age in boys | |
| Pubertal changes with associated headaches, vision changes, new-onset seizures | |
| Rapid pubertal progression | |
| Confirmed central or peripheral precocious puberty (not a generally benign variant) | |
| Known predisposing conditions (e.g., neurofibromatosis, previous irradiation, known neoplasm) | |
| Concern for delayed puberty† | |
| Boys without testicular growth to at least 4 mL in volume or 2.5 cm in length by 14 years of age | |
| Girls without breast development by 13 years of age | |
Data Sources: A PubMed search was completed using the MeSH function with the key term puberty and at least one of the following qualifiers: early, precocious, delayed, absent, or disorder. The search included meta-analyses, randomized controlled trials, observational studies, and reviews. Nonhuman studies and studies older than 10 years were excluded. The reference lists of included reviews were searched for additional studies of interest. Other searches included Essential Evidence Plus, the Cochrane Database of Systematic Reviews, and the U.S. Preventive Services Task Force website. Search dates: October 1, 2016, to May 21, 2017.
The views expressed in this publication are those of the authors and do not reflect the official policy or position of the Departments of the Army, Navy, or Air Force; the Department of Defense; or the U.S. government.
