Am Fam Physician. 2017;96(12):online
Original Article: Update on Office-Based Strategies for the Management of Obesity
Issue Date: September 1, 2016
See additional reader comments at: http://www.aafp.org/afp/2016/0901/p361.html
to the editor: In their article, Erlandson and colleagues do a good job of providing physicians with up-to-date and practical information on the outpatient management of obesity. However, in Table 4 in the row for hormones, progestins are incorrectly labeled as weight negative and estrogens are incorrectly labeled as weight positive. In a recent Cochrane review, progestin-only contraceptives (POCs) were found to be weight neutral or modestly weight positive (weight gain of about 4.4 lb [2 kg] over six to 12 months).1 Another study looking specifically at injectable progestin-only contraceptives (i.e., medroxyprogesterone [Provera]) shows more robust weight gain of 13.7 lb (6.2 kg) over five years.2 In addition to weight effects, many obese females on progestin-only contraceptives struggle with hyperandrogenic symptoms such as acne, facial hair, and dyslipidemia. These effects can be exacerbated depending on the generation of progestin used, with third-generation progestins being the most advantageous (Table 1).
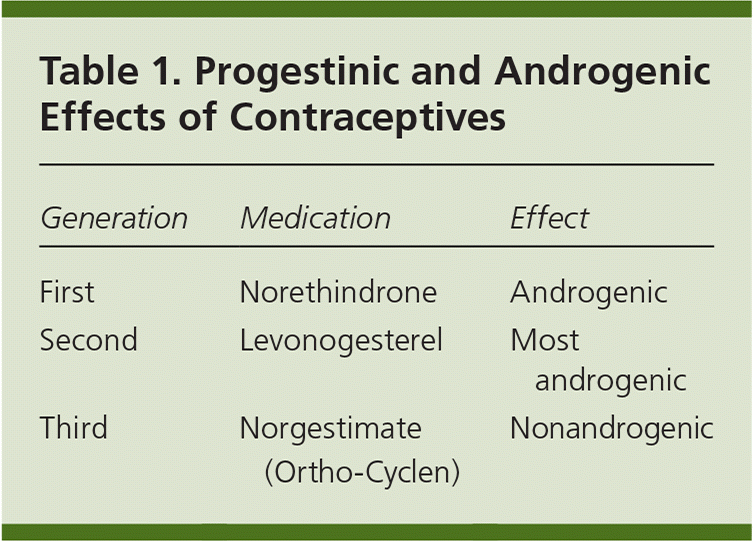
| Generation | Medication | Effect |
|---|---|---|
| First | Norethindrone | Androgenic |
| Second | Levonogesterel | Most androgenic |
| Third | Norgestimate (Ortho-Cyclen) | Nonandrogenic |
Estrogens are generally not considered weight-positive medications. In a Cochrane review of 49 trials, mixed oral contraceptives and contraceptive patches were found to be weight neutral.3 Compared with a sham method, oral contraceptives and patches did not lead to additional weight gain, a perception of additional weight gain, nor discontinuation because of weight gain.3 Family physicians should reassure their female patients about the generally weight-neutral effects of these medications. The optimal oral contraceptive for an obese patient would be an estrogen plus third-generation progestin.
in reply: I would like to thank Dr. Virji for his comments regarding the effect of estrogens and progestins on weight. Estrogens and progestins are hormonal agents that are used in a wide range of doses and combinations and for multiple indications, making generalizations about their effects difficult. Our characterization of these medications was taken from the Obesity Medicine Association's obesity algorithm, which has been updated recently to include potential weight gain with progestins.1 I agree that the cited reviews show limited effect on weight when these agents are used for contraception, despite common perceptions. In addition, systematic reviews have shown that postmenopausal hormone therapy, including unopposed estrogen and combination therapy, has no effect on weight.2,3 It does seem appropriate to classify estrogens and progestins as weight neutral, with progestins having the potential to be mildly weight positive. This is consistent with the Endocrine Society's 2015 clinical practice guideline that recommends choosing an oral contraceptive over an injectable option in women with a body mass index greater than 27 kg per m2 or greater than 30 kg per m2 in those with comorbidities.4