Am Fam Physician. 2018;97(1):29-37
Author disclosure: No relevant financial affiliations.
In patients with type 2 diabetes mellitus, insulin may be used to augment therapy with oral glycemic medications or as insulin replacement therapy. The American Diabetes Association suggests the use of long-acting (basal) insulin to augment therapy with one or two oral agents or one oral agent plus a glucagon-like peptide 1 receptor agonist when the A1C level is 9% or more, especially if the patient has symptoms of hyperglycemia or catabolism. Insulin regimens should be adjusted every three or four days until targets of self-monitored blood glucose levels are reached. A fasting and premeal blood glucose goal of 80 to 130 mg per dL and a two-hour postprandial goal of less than 180 mg per dL are recommended. Insulin use is associated with hypoglycemia and weight gain. Insulin analogues are as effective as human insulin at lowering A1C levels with lower risk of hypoglycemia, but they have significantly higher cost. Patients with one or more episodes of severe hypoglycemia (i.e., requiring assistance from others for treatment) may benefit from a short-term relaxation of glycemic targets. Several new insulin formulations have been approved recently that are associated with less risk of hypoglycemia compared with older formulations. The goals of therapy should be individualized based on many factors, including age, life expectancy, comorbid conditions, duration of diabetes, risk of hypoglycemia, cost, patient motivation, and quality of life.
Type 2 diabetes mellitus is a chronic, progressive disease characterized by multiple defects in glucose metabolism, the core of which is insulin resistance in muscle, liver, and adipocytes and progressive beta cell failure.1 Beta cell failure progresses at a rate of approximately 4% per year, requiring the use of multiple medications, often including insulin, to attain and maintain glycemic control.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Clinicians should minimize the use of concomitant medications that may cause weight gain when treating patients with insulin therapy for type 2 diabetes mellitus. | C | 6, 9–11, 33 |
| Consider initiating basal insulin to augment therapy with one or two oral agents or one oral agent plus a GLP-1 receptor agonist when the A1C is 9% or more, especially if symptoms of hyperglycemia or catabolism are present. Or, consider the addition of basal insulin to augment therapy with two oral agents with or without GLP-1 receptor agonists when the A1C is more than 8%. | C | 9, 10 |
| Consider initiating insulin replacement therapy when the blood glucose level is 300 to 350 mg per dL (16.7 to 19.4 mmol per L) or more or the A1C is more than 10% to 12%. Also consider adding rapid-acting insulin in those patients taking basal insulin who are already on augmentation therapy but not attaining A1C goals. | C | 9, 10 |
| Insulin analogues may be used to reduce the risk of hypoglycemia. | A | 28, 29 |
| The A1C goal should be individualized based on age, life expectancy, comorbid conditions, duration of diabetes, risk of hypoglycemia, adverse consequences related to hypoglycemia, or patient motivation and adherence. | C | 9, 10, 18–25 |
| Intensive control of type 2 diabetes (A1C goal below 7%) significantly decreases the need for photocoagulation treatment of diabetic retinopathy but increases hypoglycemia and mortality risk. | A | 13, 33–37 |
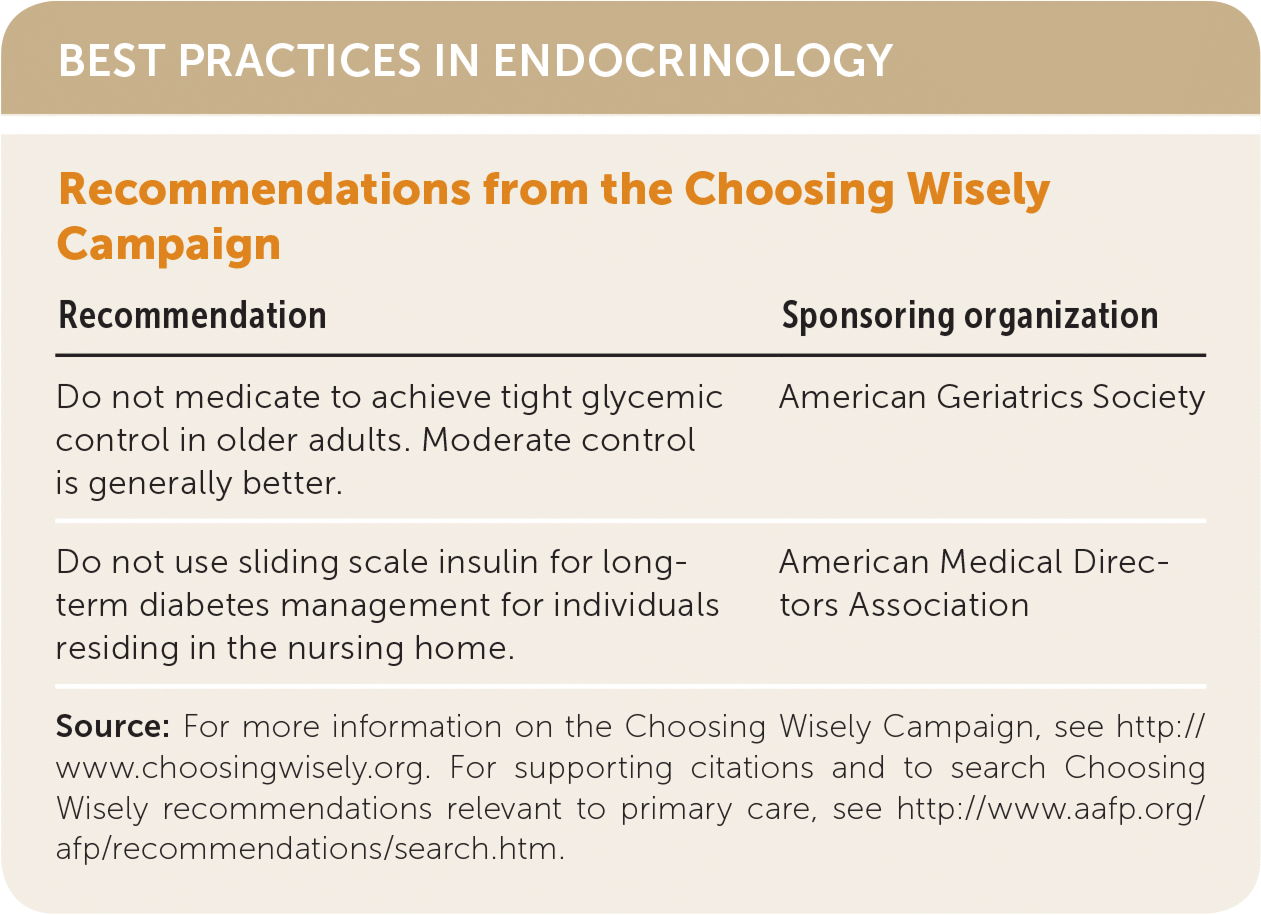
| Recommendation | Sponsoring organization |
|---|---|
| Do not medicate to achieve tight glycemic control in older adults. Moderate control is generally better. | American Geriatrics Society |
| Do not use sliding scale insulin for long-term diabetes management for individuals residing in the nursing home. | American Medical Directors Associa |
Data from the National Health and Nutrition Examination Survey show that the percentage of U.S. adults with diabetes and an A1C level of more than 9% decreased slightly between 2003 to 2006 and 2007 to 2010, from 13% to 12.6% (relative risk reduction = 3.1%; 95% confidence interval, −3.8% to –3.0%).3 According to the Centers for Disease Control and Prevention, from 2010 to 2012, 57% of patients with type 2 diabetes used only oral diabetes medications.4 Thus, it is likely that many patients who should be receiving insulin therapy are not. American Family Physician recently published a review of noninsulin therapies for type 2 diabetes.5 Our article reviews insulin management in the outpatient setting.
Concerns About Insulin Therapy
Insulin use, particularly in high doses, is associated with weight gain.6–8 American Diabetes Association (ADA) guidelines and American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) guidelines suggest minimizing the use of concomitant medications that may cause weight gain when treating patients with type 2 diabetes.9,10 Based on expert opinion, long-acting (basal) insulin combined with metformin, pramlintide (Symlin), or a glucagon-like peptide 1 (GLP-1) receptor agonist may be preferable to mitigate weight gain in obese patients compared with basal insulin alone or combined with a sulfonylurea.11
Hypoglycemia may result from a mismatch between insulin and carbohydrate intake, exercise, or alcohol consumption. Concerns about the risk of hypoglycemia can prevent or delay the initiation or intensification of insulin therapy.12 Of patients taking insulin, 7% to 15% experience at least one episode of hypoglycemia per year, and 1% to 2% have severe hypoglycemia (i.e., requiring assistance from others for treatment). Hypoglycemia has been associated with poor outcomes and higher rates of death, especially in older patients.13–17 Patients with type 2 diabetes and a history of at least one severe hypoglycemic event have an approximately two- to fourfold higher death rate than those who have not had a severe event.18–21 Hypoglycemia has also been associated with an increased risk of dementia and cardiac arrhythmias.22–24
All patients should be educated about the symptoms and self-treatment of hypoglycemia. The ADA recommends the following: (1) check the blood glucose level if signs or symptoms of hypoglycemia are present; (2) if the blood glucose level is less than 70 mg per dL (3.9 mmol per L), treat with 15 g of fast-acting carbohydrate, such as 4 oz of fruit juice or three or four glucose tablets; and (3) recheck the blood glucose level after 15 minutes to ensure that it has normalized.9
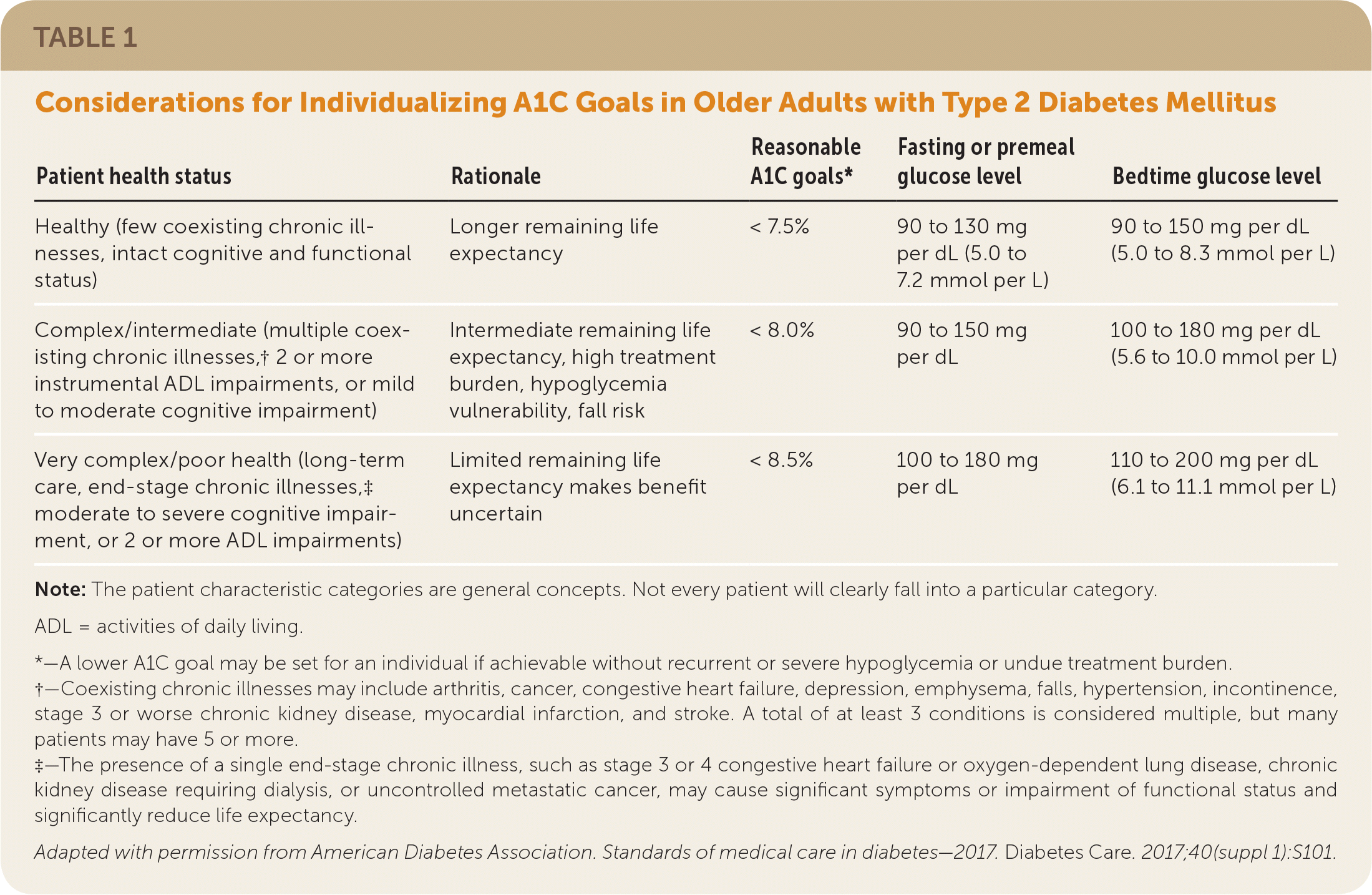
| Patient health status | Rationale | Reasonable A1C goals* | Fasting or premeal glucose level | Bedtime glucose level |
|---|---|---|---|---|
| Healthy (few coexisting chronic illnesses, intact cognitive and functional status) | Longer remaining life expectancy | < 7.5% | 90 to 130 mg per dL (5.0 to 7.2 mmol per L) | 90 to 150 mg per dL (5.0 to 8.3 mmol per L) |
| Complex/intermediate (multiple coexisting chronic illnesses,† 2 or more instrumental ADL impairments, or mild to moderate cognitive impairment) | Intermediate remaining life expectancy, high treatment burden, hypoglycemia vulnerability, fall risk | < 8.0% | 90 to 150 mg per dL | 100 to 180 mg per dL (5.6 to 10.0 mmol per L) |
| Very complex/poor health (long-term care, end-stage chronic illnesses,‡ moderate to severe cognitive impairment, or 2 or more ADL impairments) | Limited remaining life expectancy makes benefit uncertain | < 8.5% | 100 to 180 mg per dL | 110 to 200 mg per dL (6.1 to 11.1 mmol per L) |
Initiation of Insulin
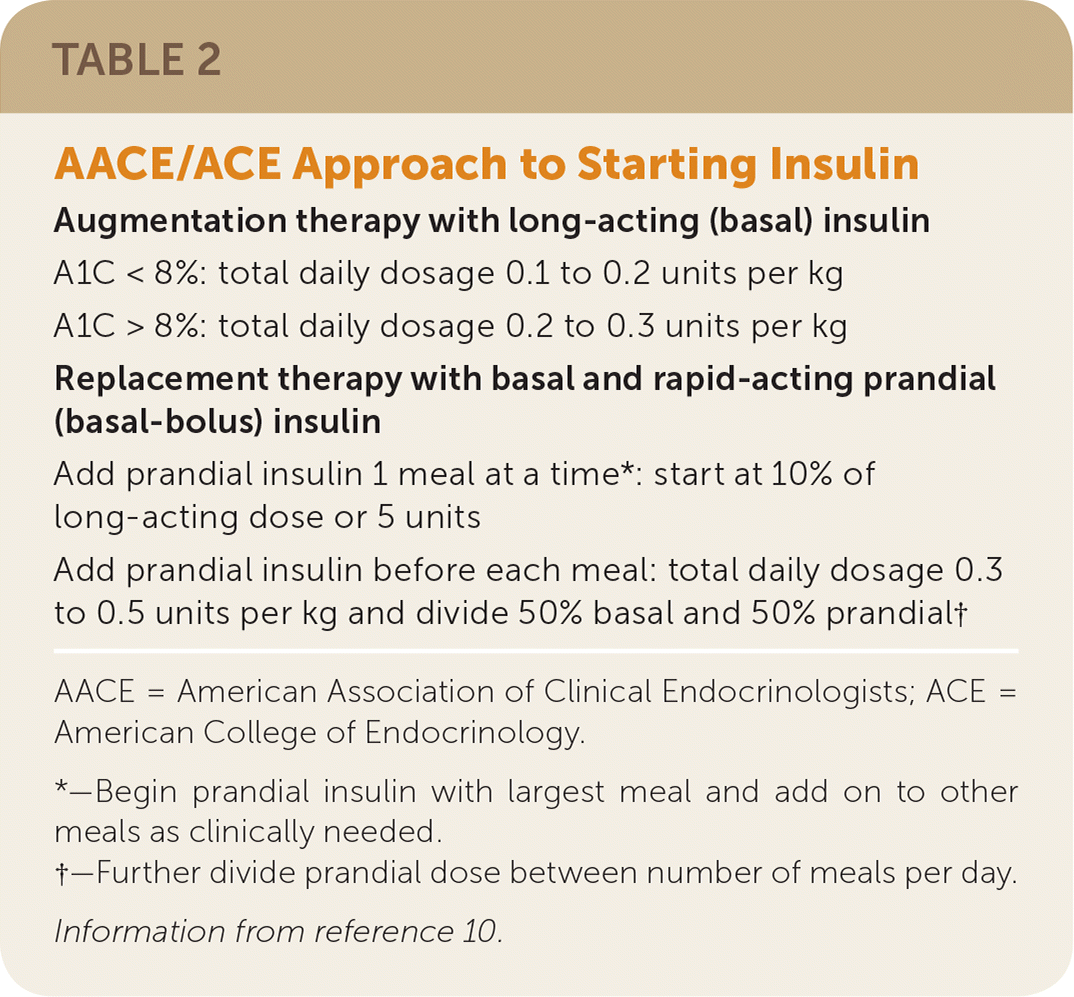
| Augmentation therapy with long-acting (basal) insulin |
| A1C < 8%: total daily dosage 0.1 to 0.2 units per kg |
| A1C > 8%: total daily dosage 0.2 to 0.3 units per kg |
| Replacement therapy with basal and rapid-acting prandial (basal-bolus) insulin |
| Add prandial insulin 1 meal at a time*: start at 10% of long-acting dose or 5 units |
| Add prandial insulin before each meal: total daily dosage 0.3 to 0.5 units per kg and divide 50% basal and 50% prandial† |
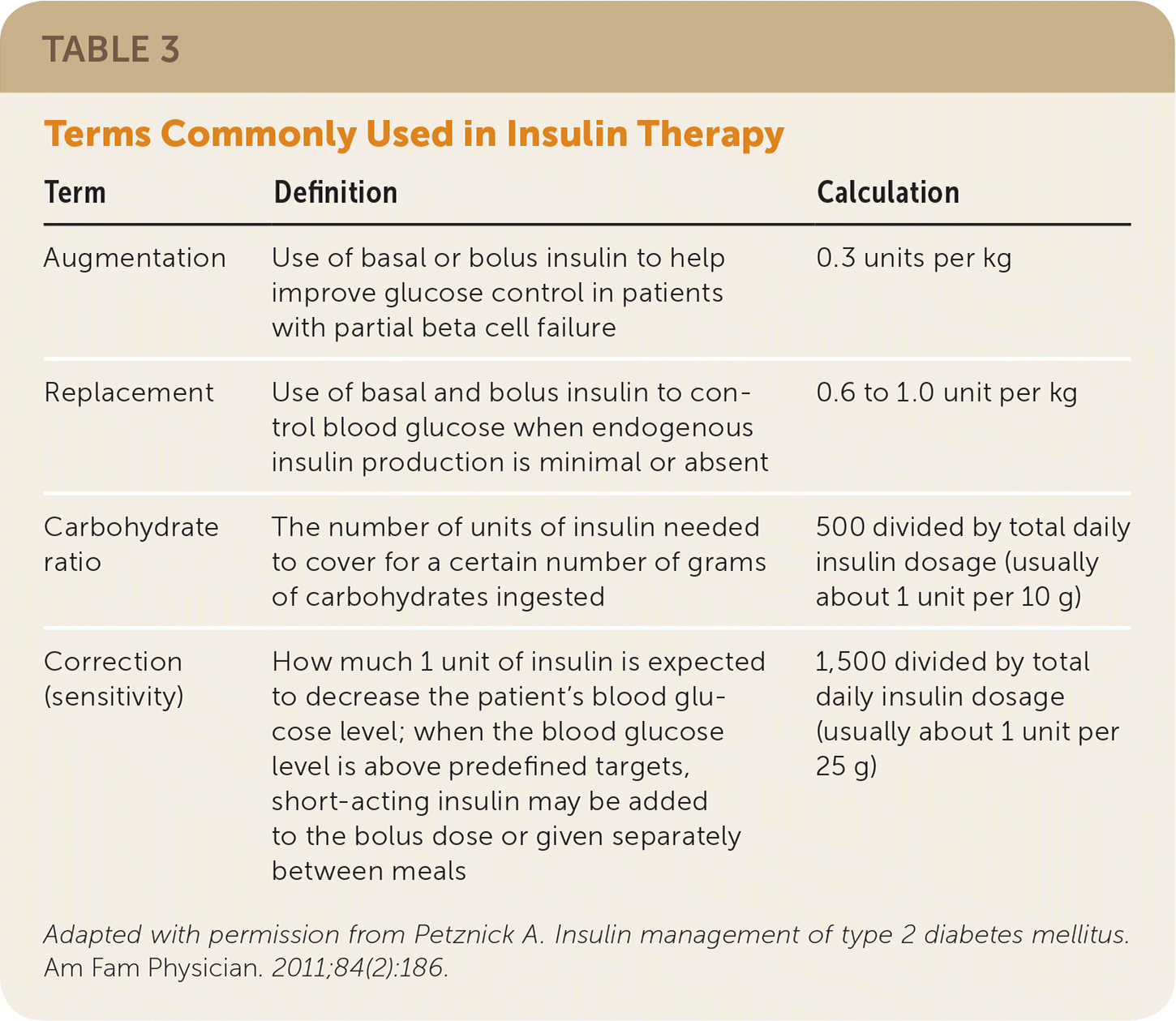
| Term | Definition | Calculation |
|---|---|---|
| Augmentation | Use of basal or bolus insulin to help improve glucose control in patients with partial beta cell failure | 0.3 units per kg |
| Replacement | Use of basal and bolus insulin to control blood glucose when endogenous insulin production is minimal or absent | 0.6 to 1.0 unit per kg |
| Carbohydrate ratio | The number of units of insulin needed to cover for a certain number of grams of carbohydrates ingested | 500 divided by total daily insulin dosage (usually about 1 unit per 10 g) |
| Correction (sensitivity) | How much 1 unit of insulin is expected to decrease the patient's blood glucose level; when the blood glucose level is above predefined targets, short-acting insulin may be added to the bolus dose or given separately between meals | 1,500 divided by total daily insulin dosage (usually about 1 unit per 25 g) |
AUGMENTATION
Augmentation therapy with basal insulin may be initiated at 10 units once daily, or by using weight-based dose calculations.9,10 The ADA suggests the use of basal insulin to augment therapy with one or two oral agents or one oral agent plus a GLP-1 receptor agonist when the A1C is 9% or more, especially if the patient has symptoms of hyperglycemia or catabolism.9 The AACE/ACE guidelines recommend the addition of basal insulin to augment therapy with two oral agents with or without GLP-1 receptor agonists when the A1C is more than 8%.10 The ADA also recommends basal insulin combined with other agents when the A1C is more than 9% at diagnosis and the patient has symptoms of hyperglycemia.10
REPLACEMENT
The ADA suggests insulin replacement therapy with basal and rapid-acting prandial (basal-bolus) insulin when the blood glucose level is 300 to 350 mg per dL (16.7 to 19.4 mmol per L) or more or the A1C is more than 10% to 12%. Insulin replacement therapy may also be considered in patients with newly diagnosed type 2 diabetes and elevated blood glucose or A1C and hyperglycemic symptoms.9,25 In patients already receiving insulin augmentation therapy who are not meeting A1C goals, the AACE/ACE guidelines suggest adding rapid-acting prandial insulin.10
Insulin analogues are as effective as human insulin at lowering A1C levels with lower risk of hypoglycemia, but they have significantly higher cost.10,27 The long-acting insulin analogues detemir (Levemir) and glargine (Lantus) cause significantly fewer nocturnal hypoglycemic events compared with isophane (NPH; Humulin N) human insulin.28 The rapid-acting insulin analogues aspart (Novolog), glulisine (Apidra), and lispro (Humalog) are associated with small but statistically significant reductions in hypoglycemic events compared with regular human insulin.29
Titration, Monitoring, and Goals of Therapy
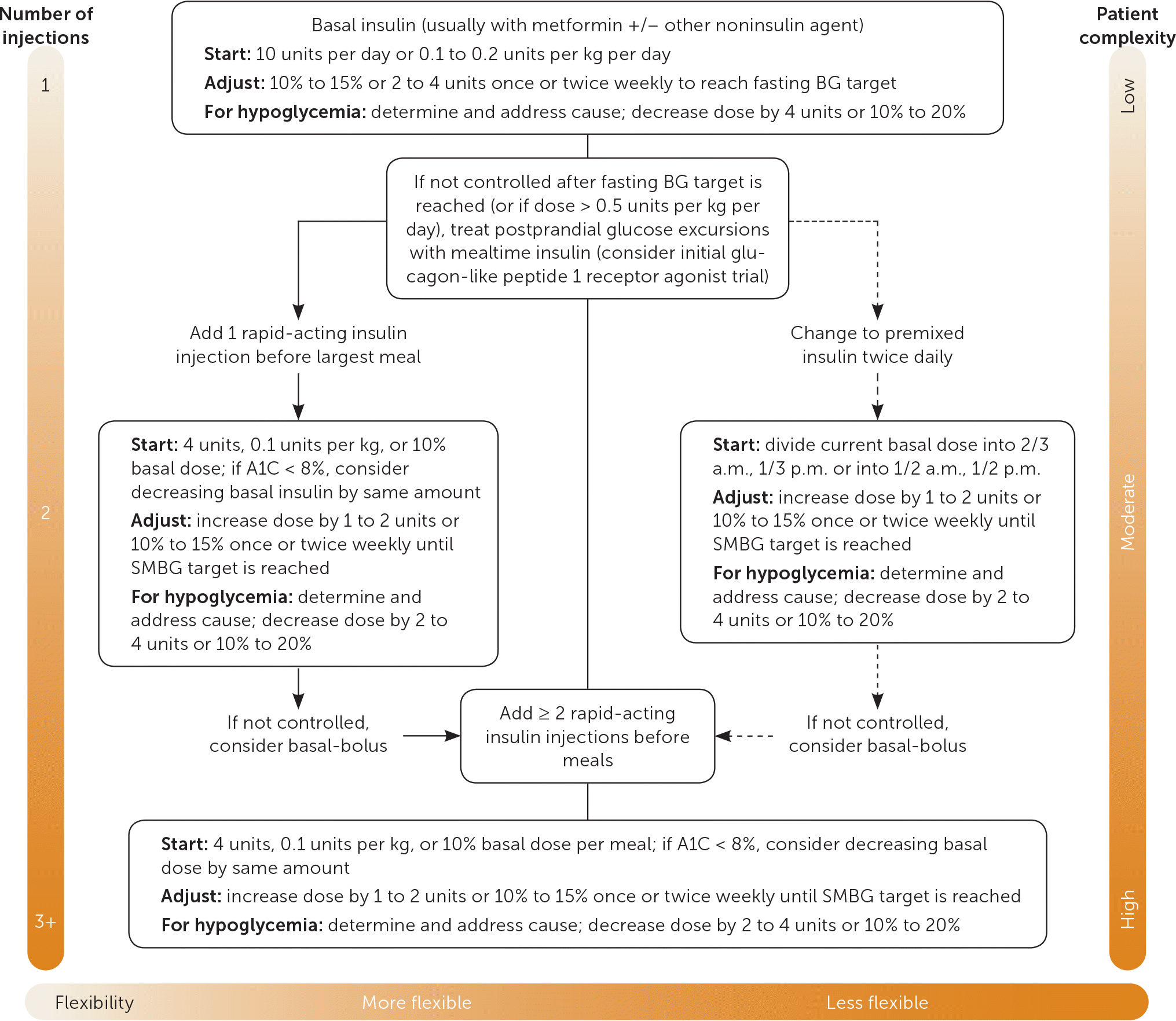
The ADA recommends that an insulin regimen be adjusted once or twice weekly (or every three or four days) until self-monitoring of blood glucose (SMBG) targets are reached.9,25 AACE/ACE guidelines differ slightly, recommending adjustment every two or three days.10 Table 430 and Figure 125 show different approaches to insulin titration depending on the type of insulin used and the resulting SMBG readings. It should be noted that these recommendations were developed before the U.S. Food and Drug Administration (FDA) approved new, highly concentrated insulins.
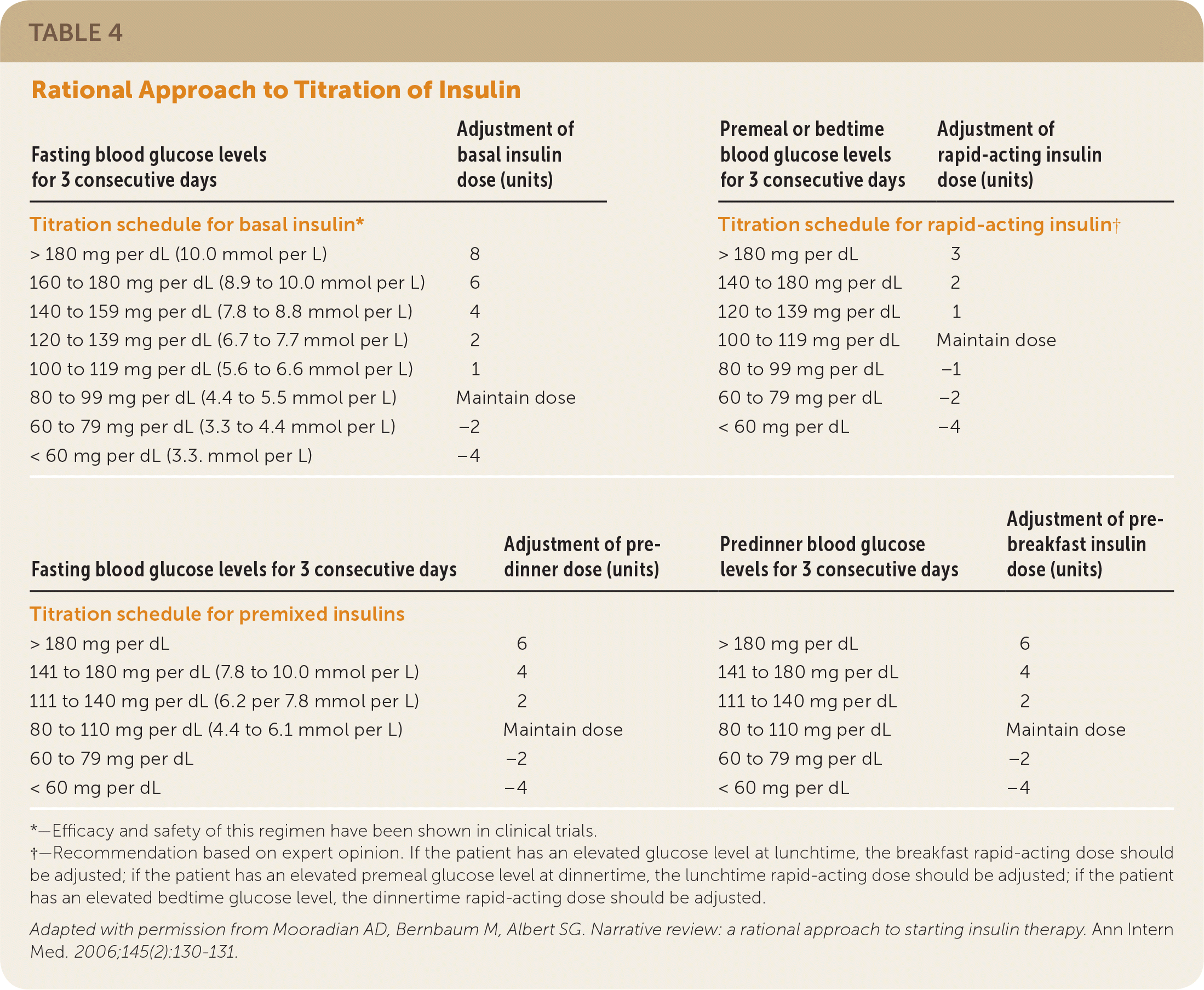
| Fasting blood glucose levels for 3 consecutive days | Adjustment of basal insulin dose (units) | Premeal or bedtime blood glucose levels for 3 consecutive days | Adjustment of rapid-acting insulin dose (units) |
| Titration schedule for basal insulin* | Titration schedule for rapid-acting insulin† | ||
| > 180 mg per dL (10.0 mmol per L) | 8 | > 180 mg per dL | 3 |
| 160 to 180 mg per dL (8.9 to 10.0 mmol per L) | 6 | 140 to 180 mg per dL | 2 |
| 140 to 159 mg per dL (7.8 to 8.8 mmol per L) | 4 | 120 to 139 mg per dL | 1 |
| 120 to 139 mg per dL (6.7 to 7.7 mmol per L) | 2 | 100 to 119 mg per dL | Maintain dose |
| 100 to 119 mg per dL (5.6 to 6.6 mmol per L) | 1 | 80 to 99 mg per dL | −1 |
| 80 to 99 mg per dL (4.4 to 5.5 mmol per L) | Maintain dose | 60 to 79 mg per dL | −2 |
| 60 to 79 mg per dL (3.3 to 4.4 mmol per L) | −2 | < 60 mg per dL | −4 |
| < 60 mg per dL (3.3. mmol per L) | −4 | ||
| Fasting blood glucose levels for 3 consecutive days | Adjustment of predinner dose (units) | Predinner blood glucose levels for 3 consecutive days | Adjustment of prebreakfast insulin dose (units) |
| Titration schedule for premixed insulins | |||
| > 180 mg per dL | 6 | > 180 mg per dL | 6 |
| 141 to 180 mg per dL (7.8 to 10.0 mmol per L) | 4 | 141 to 180 mg per dL | 4 |
| 111 to 140 mg per dL (6.2 per 7.8 mmol per L) | 2 | 111 to 140 mg per dL | 2 |
| 80 to 110 mg per dL (4.4 to 6.1 mmol per L) | Maintain dose | 80 to 110 mg per dL | Maintain dose |
| 60 to 79 mg per dL | −2 | 60 to 79 mg per dL | −2 |
| < 60 mg per dL | −4 | < 60 mg per dL | −4 |
The ADA suggests that patients taking multiple daily insulin injections consider performing SMBG before meals and snacks, occasionally after meals, at bedtime, before exercise, when hypoglycemia is suspected and after it is treated, and before critical tasks such as driving.9 Reduced A1C levels have also been demonstrated in patients taking only basal insulin who perform fasting blood glucose testing and achieve their fasting blood glucose goal.31 Performing more frequent SMBG may be helpful; one study showed that increased frequency is associated with better glycemic control.32 The ADA recommends a fasting and premeal SMBG goal of 80 to 130 mg per dL (4.4 to 7.2 mmol per L) and a two-hour postprandial goal of less than 180 mg per dL (10.0 mmol per L).9 The AACE/ACE guidelines recommend a fasting and premeal SMBG goal of 70 to 110 mg per dL (3.9 to 6.1 mmol per L) and a two-hour postprandial goal of less than 140 mg per dL (7.8 mmol per L).10 The A1C should be tested every three months in patients with unstable or uncontrolled type 2 diabetes. Twice yearly measurements are reasonable for patients with stable diabetes and an A1C level that is within the goal.9
A1C goals should be individualized according to patient characteristics.9,10 The U.K. Prospective Diabetes Study showed that patients with newly diagnosed type 2 diabetes who received intensive therapy (median A1C of 7% achieved) with sulfonylureas, insulin, or metformin had a significantly lower risk of microvascular complications compared with patients who received conventional therapy (median A1C of 7.9% achieved).33,34 However, most of the benefits in this study were due to a reduced need for photocoagulation treatment of diabetic retinopathy. Several recent trials of intensive therapy in older patients with long duration of diabetes and a high risk of atherosclerotic cardiovascular disease have shown no effect on the incidence of macrovascular complications or an increased incidence of hypoglycemic events and increased mortality.13,35–37
The ADA suggests a target A1C of less than 7% for most nonpregnant patients with type 2 diabetes. An A1C goal of less than 6.5% may be appropriate for patients with short duration of type 2 diabetes that is treated with lifestyle changes or metformin only, a long life expectancy, and no significant cardiovascular disease, as long as significant hypoglycemia or other adverse effects do not occur. For patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbidities, or long duration of type 2 diabetes, an A1C goal of less than 8% or more may be appropriate (Table 1).9
New Insulin Products
Table 5 summarizes FDA-approved insulin products; see eTable A and eTable B for information about pharmacokinetics and shelf life. Before 2015, the standard concentration for all insulin formulations was U-100, with the exception of the U-500 regular human insulin Humulin R. Since then, the FDA has approved several higher-concentration insulin products that are associated with less risk of hypoglycemia compared with older formulations.
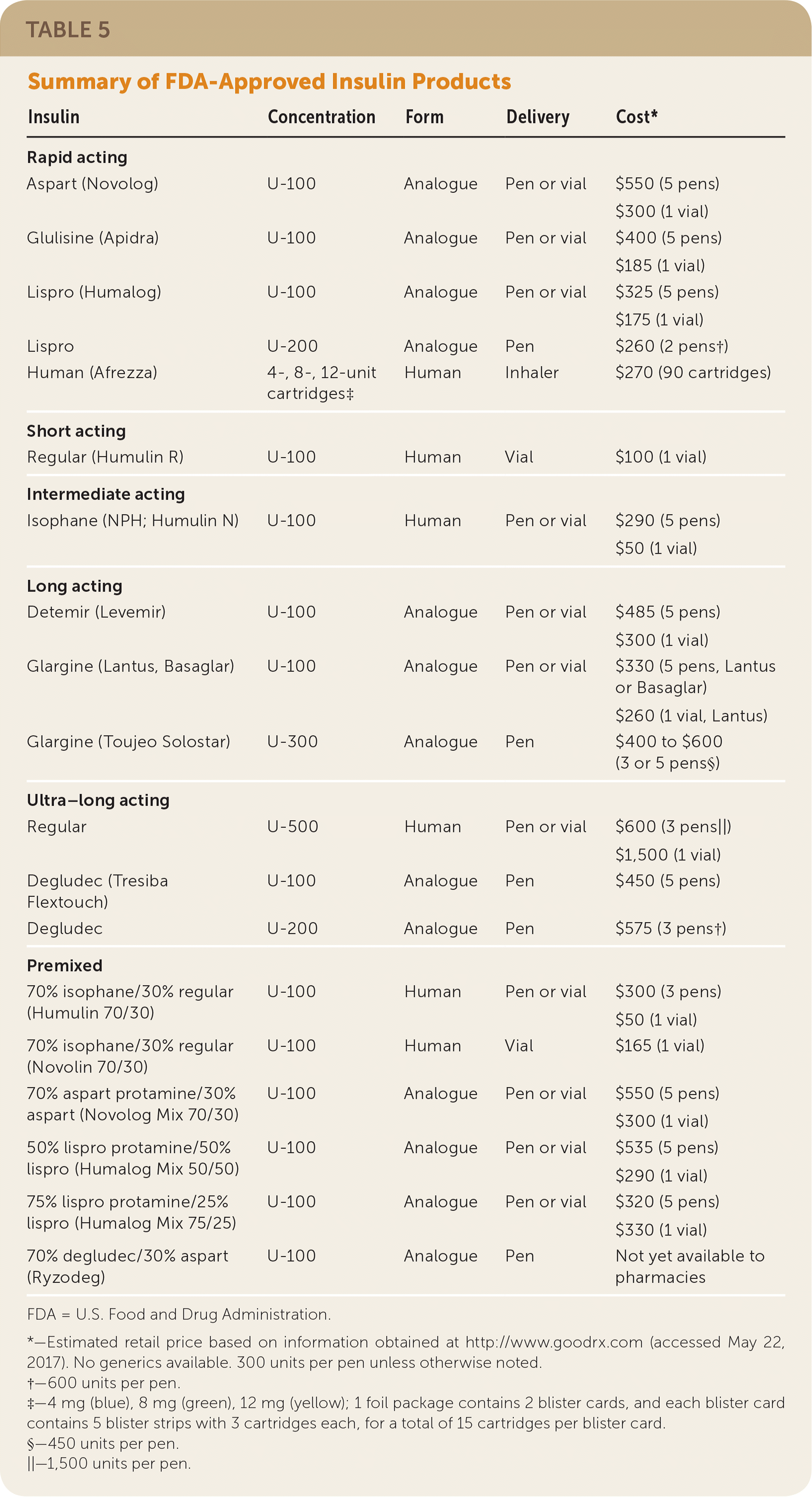
| Insulin | Concentration | Form | Delivery | Cost* |
|---|---|---|---|---|
| Rapid acting | ||||
| Aspart (Novolog) | U-100 | Analogue | Pen or vial | $550 (5 pens) $300 (1 vial) |
| Glulisine (Apidra) | U-100 | Analogue | Pen or vial | $400 (5 pens) $185 (1 vial) |
| Lispro (Humalog) | U-100 | Analogue | Pen or vial | $325 (5 pens) $175 (1 vial) |
| Lispro | U-200 | Analogue | Pen | $260 (2 pens†) |
| Human (Afrezza) | 4-, 8-, 12-unit cartridges‡ | Human | Inhaler | $270 (90 cartridges) |
| Short acting | ||||
| Regular (Humulin R) | U-100 | Human | Vial | $100 (1 vial) |
| Intermediate acting | ||||
| Isophane (NPH; Humulin N) | U-100 | Human | Pen or vial | $290 (5 pens) $50 (1 vial) |
| Long acting | ||||
| Detemir (Levemir) | U-100 | Analogue | Pen or vial | $485 (5 pens) $300 (1 vial) |
| Glargine (Lantus, Basaglar) | U-100 | Analogue | Pen or vial | $330 (5 pens, Lantus or Basaglar) $260 (1 vial, Lantus) |
| Glargine (Toujeo Solostar) | U-300 | Analogue | Pen | $400 to $600 (3 or 5 pens§) |
| Ultra–long acting | ||||
| Regular | U-500 | Human | Pen or vial | $600 (3 pens||) $1,500 (1 vial) |
| Degludec (Tresiba Flextouch) | U-100 | Analogue | Pen | $450 (5 pens) |
| Degludec | U-200 | Analogue | Pen | $575 (3 pens†) |
| Premixed | ||||
| 70% isophane/30% regular (Humulin 70/30) | U-100 | Human | Pen or vial | $300 (3 pens) $50 (1 vial) |
| 70% isophane/30% regular (Novolin 70/30) | U-100 | Human | Vial | $165 (1 vial) |
| 70% aspart protamine/30% aspart (Novolog Mix 70/30) | U-100 | Analogue | Pen or vial | $550 (5 pens) $300 (1 vial) |
| 50% lispro protamine/50% lispro (Humalog Mix 50/50) | U-100 | Analogue | Pen or vial | $535 (5 pens) $290 (1 vial) |
| 75% lispro protamine/25% lispro (Humalog Mix 75/25) | U-100 | Analogue | Pen or vial | $320 (5 pens) $330 (1 vial) |
| 70% degludec/30% aspart (Ryzodeg) | U-100 | Analogue | Pen | Not yet available to pharmacies |
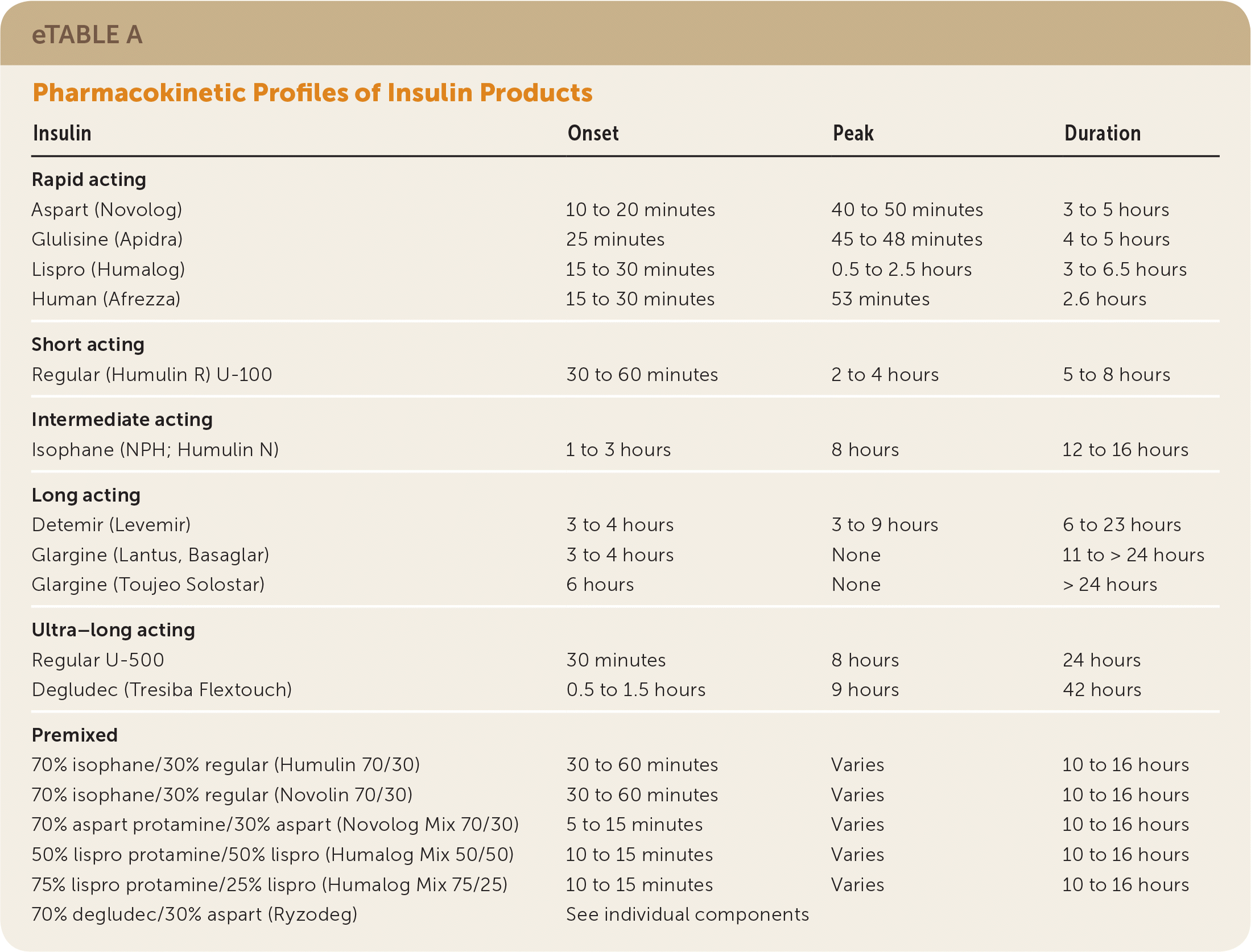
| Insulin | Onset | Peak | Duration | |
|---|---|---|---|---|
| Rapid acting | ||||
| Aspart (Novolog) | 10 to 20 minutes | 40 to 50 minutes | 3 to 5 hours | |
| Glulisine (Apidra) | 25 minutes | 45 to 48 minutes | 4 to 5 hours | |
| Lispro (Humalog) | 15 to 30 minutes | 0.5 to 2.5 hours | 3 to 6.5 hours | |
| Human (Afrezza) | 15 to 30 minutes | 53 minutes | 2.6 hours | |
| Short acting | ||||
| Regular (Humulin R) U-100 | 30 to 60 minutes | 2 to 4 hours | 5 to 8 hours | |
| Intermediate acting | ||||
| Isophane (NPH; Humulin N) | 1 to 3 hours | 8 hours | 12 to 16 hours | |
| Long acting | ||||
| Detemir (Levemir) | 3 to 4 hours | 3 to 9 hours | 6 to 23 hours | |
| Glargine (Lantus, Basaglar) | 3 to 4 hours | None | 11 to > 24 hours | |
| Glargine (Toujeo Solostar) | 6 hours | None | > 24 hours | |
| Ultra–long acting | ||||
| Regular U-500 | 30 minutes | 8 hours | 24 hours | |
| Degludec (Tresiba Flextouch) | 0.5 to 1.5 hours | 9 hours | 42 hours | |
| Premixed | ||||
| 70% isophane/30% regular (Humulin 70/30) | 30 to 60 minutes | Varies | 10 to 16 hours | |
| 70% isophane/30% regular (Novolin 70/30) | 30 to 60 minutes | Varies | 10 to 16 hours | |
| 70% aspart protamine/30% aspart (Novolog Mix 70/30) | 5 to 15 minutes | Varies | 10 to 16 hours | |
| 50% lispro protamine/50% lispro (Humalog Mix 50/50) | 10 to 15 minutes | Varies | 10 to 16 hours | |
| 75% lispro protamine/25% lispro (Humalog Mix 75/25) | 10 to 15 minutes | Varies | 10 to 16 hours | |
| 70% degludec/30% aspart (Ryzodeg) | See individual components | |||
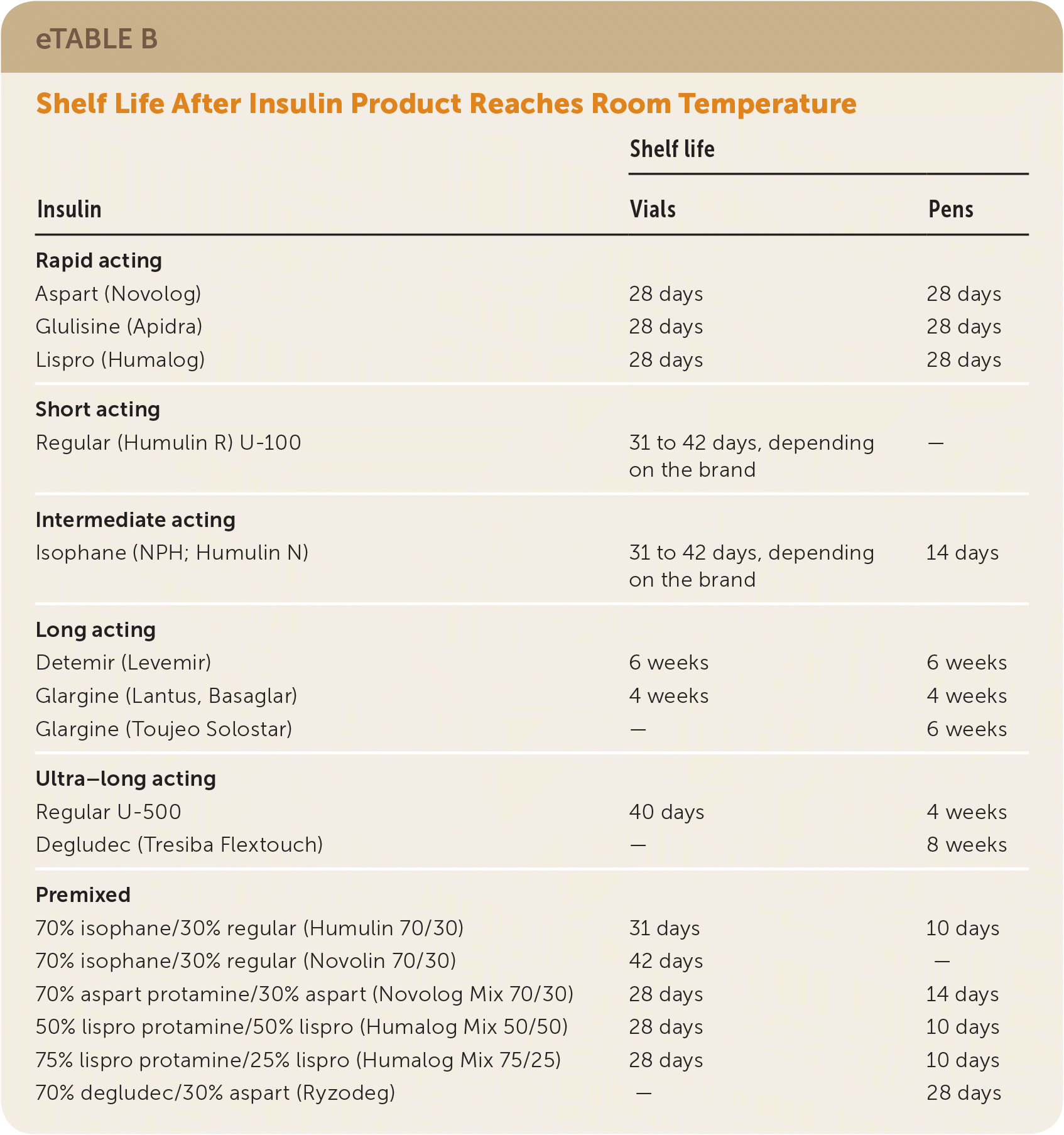
| Insulin | Shelf life | ||
|---|---|---|---|
| Vials | Pens | ||
| Rapid acting | |||
| Aspart (Novolog) | 28 days | 28 days | |
| Glulisine (Apidra) | 28 days | 28 days | |
| Lispro (Humalog) | 28 days | 28 days | |
| Short acting | |||
| Regular (Humulin R) U-100 | 31 to 42 days, depending on the brand | — | |
| Intermediate acting | |||
| Isophane (NPH; Humulin N) | 31 to 42 days, depending on the brand | 14 days | |
| Long acting | |||
| Detemir (Levemir) | 6 weeks | 6 weeks | |
| Glargine (Lantus, Basaglar) | 4 weeks | 4 weeks | |
| Glargine (Toujeo Solostar) | — | 6 weeks | |
| Ultra–long acting | |||
| Regular U-500 | 40 days | 4 weeks | |
| Degludec (Tresiba Flextouch) | — | 8 weeks | |
| Premixed | |||
| 70% isophane/30% regular (Humulin 70/30) | 31 days | 10 days | |
| 70% isophane/30% regular (Novolin 70/30) | 42 days | — | |
| 70% aspart protamine/30% aspart (Novolog Mix 70/30) | 28 days | 14 days | |
| 50% lispro protamine/50% lispro (Humalog Mix 50/50) | 28 days | 10 days | |
| 75% lispro protamine/25% lispro (Humalog Mix 75/25) | 28 days | 10 days | |
| 70% degludec/30% aspart (Ryzodeg) | — | 28 days | |
RAPID- AND SHORT-ACTING INSULINS
Afrezza, a rapid-acting inhaled human insulin, is delivered through a specially designed inhaler comprised of single-use cartridges.40 eTable C shows dose conversions from rapid-acting U-100 injectable insulin to Afrezza. In January 2016, Sanofi-Aventis announced it would no longer distribute Afrezza because of poor sales, and it is unclear whether Afrezza will remain on the market in the United States.40,41
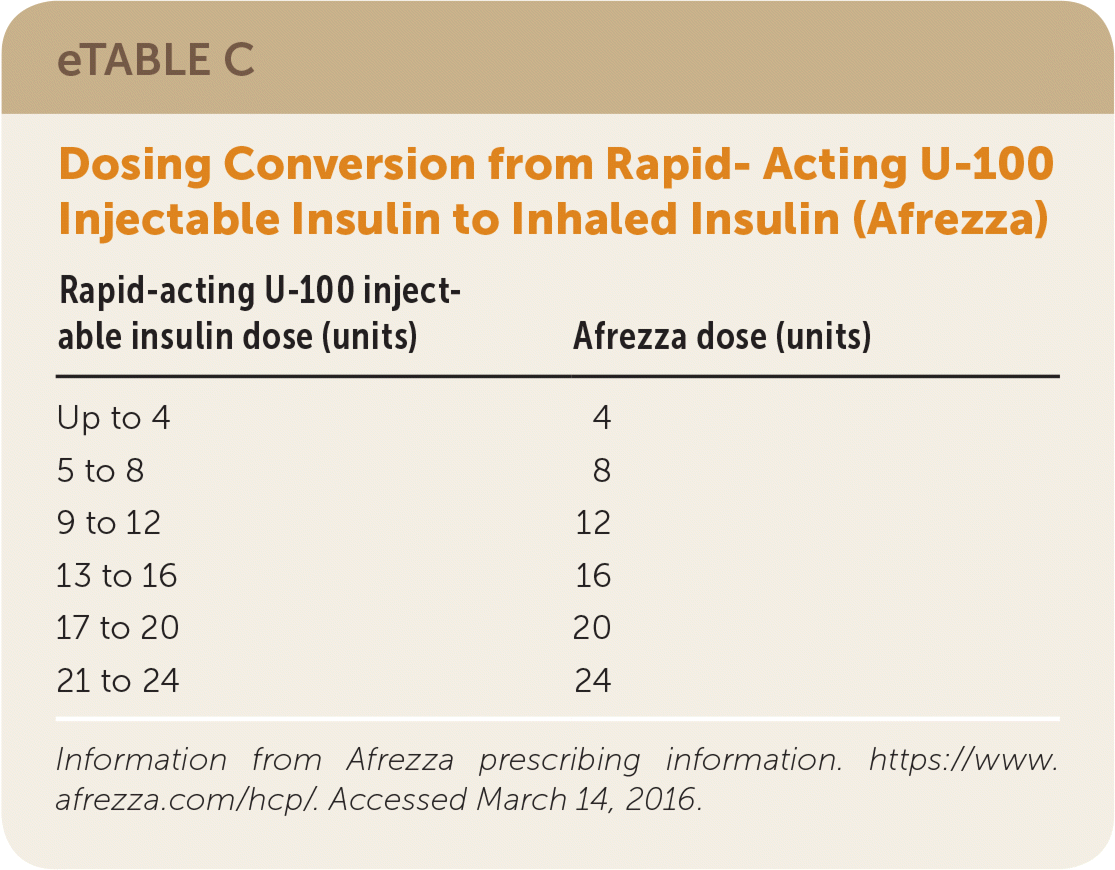
| Rapid-acting U-100 injectable insulin dose (units) | Afrezza dose (units) |
|---|---|
| Up to 4 | 4 |
| 5 to 8 | 8 |
| 9 to 12 | 12 |
| 13 to 16 | 16 |
| 17 to 20 | 20 |
| 21 to 24 | 24 |
U-500 regular human insulin is now available as a pen. This product is indicated for patients whose total dosage of U-100 insulin is more than 200 units per day. Unlike the vial formulation, the pen does not require a dose conversion when switching from U-100 insulin formulations. Dose adjustments must be made in 5-unit increments because of the dose increments available in the pen. U-500 regular human insulin is the most concentrated formulation of insulin available as a pen and allows for the administration of the largest number of insulin units per injection.42 U-500 regular human insulin has been shown to improve glycemic control in severely insulin-resistant patients who require large doses of U-100 insulin. It is also associated with increased patient satisfaction and reduced cost.43
LONG-ACTING INSULINS
The U-300 glargine pen (Toujeo Solostar) is a long-acting analogue insulin that was developed to reduce injection volume and provide a gradual insulin release to increase its duration of action to more than 24 hours.44 Toujeo Solostar causes significantly fewer hypoglycemic events at any time of day and nocturnally compared with U-100 glargine (Lantus) in patients with type 2 diabetes.45 Toujeo Solostar lowers A1C as effectively as Lantus after six months of treatment and marginally better than Lantus after 12 months.46 Toujeo Solostar reaches steady-state concentration within five days; therefore, the dose should be titrated no more than once every three or four days. Toujeo Solostar allows dosing with 1-unit increments with even numbers visible in the dose counter window.44
The insulin glargine pen Basaglar was approved by the FDA in December 2015 as the first “follow-on” insulin. Although its amino acid sequence is identical to Lantus, it is not considered a biosimilar product in the United States because it did not go through the FDA's biosimilar drug approval process.47
The degludec pen (Tresiba Flextouch) is an ultra–long-acting analogue insulin that is available in two concentrations (U-100 and U-200) with similar safety, effectiveness, and pharmacokinetic profiles. Dose conversion is not required when switching between the two.48,49 Tresiba Flextouch has a longer duration of action than Toujeo Solostar or Lantus. Patients inject Tresiba Flextouch once daily at any time, but there must be at least eight hours between injections.50 Both concentrations of Tresiba Flextouch lower A1C with a similar effectiveness as U-100 glargine (Lantus) with less overall and nocturnal hypoglycemia.51–56 U-200 Tresiba Flextouch shows only even numbers in the dose counter window, requiring all doses to be even numbered.57
Tresiba Flextouch can be combined in solution with rapid-acting insulin. It is premixed as 70% U-100 degludec and 30% U-100 aspart (Ryzodeg). This combination has been shown to provide similar reduction in A1C values while significantly lowering the risk of overall confirmed, nocturnal confirmed, and severe hypoglycemia.58 This product should be injected once daily with the main meal at the same dose as the previously used long-acting insulin. Patients should continue their previously used short- or rapid-acting insulin at the same dose before meals not covered by this product.59
This article updates previous articles on this topic by Petznick,26 and by Mayfield and White.60
Data Sources: We searched PubMed using the following terms: type 2 diabetes treatment, prediabetes treatment, hypoglycemic agents, nutrition and diabetes, diabetes and cardiovascular disease. We also searched Essential Evidence Plus. Search dates: January 2016 and July 2017.