Am Fam Physician. 2018;97(1):56-58
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• An IGRA is recommended over a TST in persons at least five years of age who are likely to have M. tuberculosis infection
• If pulmonary TB is suspected, an AFB smear can be performed; three specimens are typically tested.
• If extrapulmonary TB is suspected, specimens should be collected from those sites for mycobacterial culture.
From the AFP Editors
Persons with Mycobacterium tuberculosis infection may have no clinical evidence of disease and present asymptomatically, known as latent tuberculosis infection (LTBI) or symptomatically, known as tuberculosis (TB). TB, which is a chief cause of infection-related morbidity and mortality, can be difficult to diagnose. For this reason, the American Thoracic Society (ATS), Infectious Diseases Society of America (IDSA), and Centers for Disease Control and Prevention (CDC) have provided guidance on diagnosing TB in children and adults.
Recommendations
LTBI TESTING
LTBI testing recommendations are outlined in Table 1. It should be noted that although interferon-gamma release assays (IGRAs) and tuberculin skin tests (TSTs) can identify M. tuberculosis infection, they cannot differentiate between TB and LTBI; therefore, active TB needs to be excluded via presence or absence of symptoms or signs on radiography before initiating LTBI treatment.
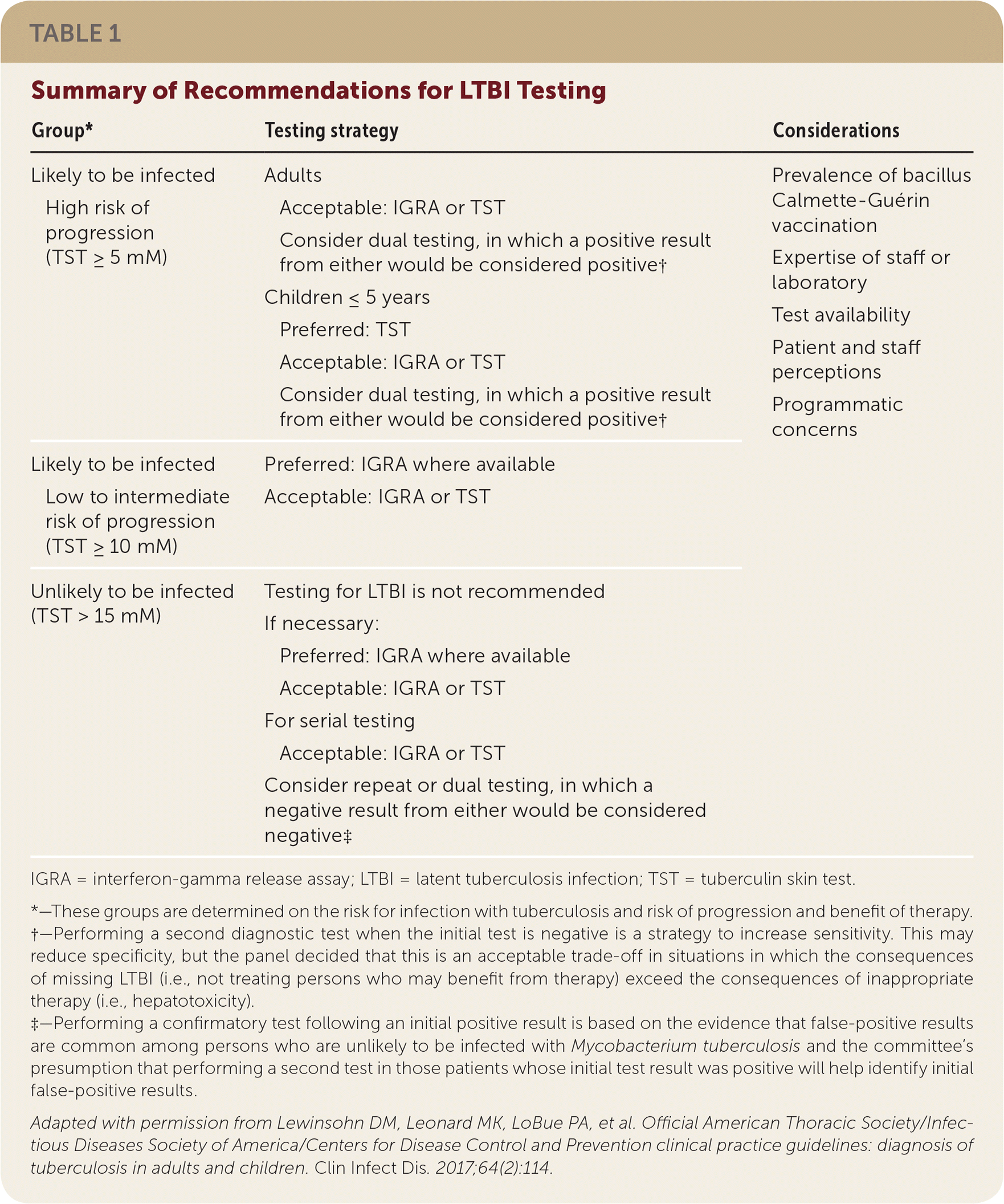
| Group* | Testing strategy | Considerations | ||
|---|---|---|---|---|
| Likely to be infected | Adults | Prevalence of bacillus Calmette-Guérin vaccination | ||
| High risk of progression (TST ≥ 5 mM) | Acceptable: IGRA or TST | Expertise of staff or laboratory | ||
| Consider dual testing, in which a positive result from either would be considered positive† | Test availability | |||
| Children ≤ 5 years | Patient and staff perceptions | |||
| Preferred: TST | Programmatic concerns | |||
| Acceptable: IGRA or TST | ||||
| Consider dual testing, in which a positive result from either would be considered positive† | ||||
| Likely to be infected | Preferred: IGRA where available | |||
| Low to intermediate risk of progression (TST ≥ 10 mM) | Acceptable: IGRA or TST | |||
| Unlikely to be infected (TST > 15 mM) | Testing for LTBI is not recommended | |||
| If necessary: | ||||
| Preferred: IGRA where available | ||||
| Acceptable: IGRA or TST | ||||
| For serial testing | ||||
| Acceptable: IGRA or TST | ||||
| Consider repeat or dual testing, in which a negative result from either would be considered negative‡ | ||||
Strong. An IGRA is recommended over a TST in persons at least five years of age who are likely to have M. tuberculosis infection; who are at low or moderate risk of the disease progressing; in whom it has been determined that LTBI testing is necessary; and who have been vaccinated against bacillus Calmette-Guérin or are not likely to return for follow-up after a TST. The TST is a viable second option in certain circumstances, such as if an IGRA is unavailable.
Conditional. An IGRA is recommended over a TST in persons at least five years of age who are likely to have M. tuberculosis infection; are at low or moderate risk of the disease progressing; and in whom it has been determined that LTBI testing is necessary. As previously mentioned, a TST is a viable second option. A TST is recommended over IGRA in healthy children younger than five years if it has been determined that LTBI testing is necessary.
Although recommendations from other groups indicate that testing for M. tuberculosis infection is not necessary in low-risk persons, it may still be required by local law or for credentialing. For this population, an IGRA is recommended over a TST in persons at least five years of age, with a second test (i.e., IGRA or TST) performed if the result on the first test is positive. Infection is confirmed if results on both tests are positive. In certain circumstances, TST is a viable second option to be performed initially.
Unrated. Evidence is lacking to recommend TST or IGRA over the other as the first-line test in persons at least five years of age who likely have M. tuberculosis infection and a high risk of the disease progressing, and in whom it has been determined that LTBI testing is necessary.
TB TESTING
Strong. If pulmonary TB is suspected, an acid-fast bacilli (AFB) smear can be performed; three specimens are typically tested. False results are common with AFB smears; therefore, a negative result does not exclude pulmonary TB, nor does a positive result confirm it. If extrapulmonary TB is suspected, specimens should be collected from those sites for mycobacterial culture; a positive result can be reasonable evidence of disease, but a negative result does not exclude disease because of the high false-negative rate.
Rapid molecular drug susceptibility testing of respiratory specimens for rifampin alone or combined with isoniazid should be performed in persons with positive results on an AFB smear or a nucleic acid amplification test (NAAT) who have been treated previously for TB; who were born in or lived for at least one year in a foreign country with an intermediate incidence of TB or high prevalence of multidrug-resistant TB; who are in contact with persons with multidrug-resistant TB; or who have human immunodeficiency virus infection.
When there is a positive result on mycobacterial culture, one culture isolate should be provided to a regional laboratory for genotyping.
Conditional. If TB is suspected, liquid and solid mycobacterial cultures can both be performed on respiratory specimens, rather than just one or the other, for each specimen.
If pulmonary TB is suspected, NAAT is recommended for the first respiratory specimen; a negative result in a patient who had a positive result on AFB smear makes TB improbable. A positive result on NAAT in a patient who had a negative result on AFB smear, but in whom there is a moderate to high level of suspicion for TB, can be considered reasonable evidence of TB. However, a negative NAAT result does not exclude pulmonary TB. If pulmonary TB is suspected in a child, mycobacterial culture of respiratory specimens is recommended.
For adults with suspected pulmonary TB who cannot provide sputum or whose sputum was negative on AFB smear, sputum induction is recommended over flexible bronchoscopy for initial sampling. When sputum induction does not work, flexible bronchoscopy is recommended over no sampling. When adults with suspected pulmonary TB undergo bronchoscopy, sputum samples should be collected to use in AFB smears and mycobacterial cultures.
For adults with suspected miliary TB, but in whom sputum cannot be induced or whose sputum was negative on AFB smear, flexible bronchoscopy is preferred to no sampling if no other lesions can be accessed.
In persons with suspected extrapulmonary TB, cell counts and chemistries should be performed on fluid specimens (e.g., pleural or cerebrospinal fluid). An AFB smear and NAAT are also recommended; a positive result on either can be reasonable evidence of disease, but a negative result on either cannot exclude disease, because of the high false-negative rate. Histologic examination should be performed, with positive and negative results evaluated based on each individual situation.
In persons with suspected pleural, peritoneal, or pericardial TB, or tuberculous meningitis, measurement of adenosine deaminase is recommended, with measurement of free interferon gamma also performed in those with suspected pleural or peritoneal TB.
Guideline source: American Thoracic Society, Infectious Diseases Society of America, and Centers for Disease Control and Prevention
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: Clin Infect Dis. January 15, 2017;64(2):111–115