
A more recent article on buprenorphine treatment for opioid use disorder is available.
Am Fam Physician. 2018;97(5):313-320
Related editorial: Treating Opioid Use Disorders as a Family Physician: Taking the Next Step
Related editorial: How Family Physicians Can Combat the Opioid Epidemic
See related article in FPM: Preparations for Treating Opioid Use Disorder in the Office
Patient information: See related handout on opioid use disorder, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Opioid misuse, including the use of heroin and the overprescribing, misuse, and diversion of opioid pain medications, has reached epidemic proportions in the United States. As a result, there has been a dramatic increase in opioid use disorder and associated overdoses and deaths. Addiction is a chronic brain disease with a genetic component that affects motivation, inhibition, and cognition. Patient characteristics associated with successful buprenorphine maintenance treatment include stable or controlled medical or psychiatric comorbidities and a safe, substance-free environment. As a partial opioid agonist, buprenorphine has a ceiling effect that limits respiratory depression and adds to its safety in accidental or intentional overdose. Buprenorphine and combinations of buprenorphine and naloxone are generally well tolerated; adverse effects include anxiety, constipation, dizziness, drowsiness, headache, nausea, and sedation. Family physicians who meet specific requirements can obtain a Drug Addiction Treatment Act of 2000 waiver by notifying the Substance Abuse and Mental Health Services Administration of their intent to begin dispensing and/or prescribing buprenorphine. Medication-assisted treatment with buprenorphine is as effective as methadone in terms of treatment retention and decreased opioid use when prescribed at fixed dosages of at least 7 mg per day; dosages of 16 mg per day are clearly superior to placebo. Sporadic opioid use is not uncommon in the first few months of medication-assisted treatment and should be addressed by increased visit frequency and more intensive engagement with behavioral therapies. Follow-up visits should include documentation of any relapses, reemergence of cravings or withdrawal, random urine drug testing, pill or wrapper counts, and checks of state prescription drug database records.
Opioid misuse is an epidemic in the United States. In 2016, approximately 11.5 million Americans 12 years and older misused opioid pain medications, and 1.8 million had a substance use disorder involving prescription pain medications.1 From 2000 to 2015, more than 500,000 persons died from opioid overdoses, with deaths generally increasing as prescription opioid sales increased.2 In 2012, clinicians wrote 259 million prescriptions for opioids, enough for every U.S. adult.3 Primary care physicians are the largest prescribers of opioids and have an important role in reversing these negative trends.4 Although most family physicians prescribe opioids, few prescribe buprenorphine medication-assisted therapy (MAT).5 This barrier to patient access is particularly stark in rural areas.6
WHAT IS NEW ON THIS TOPIC
Buprenorphine Therapy
From 2000 to 2015, more than 500,000 persons died from opioid overdoses, with deaths generally increasing as prescription opioid sales increased.
The Diagnostic and Statistical Manual of Mental Disorders, 5th ed., replaced the older terms opioid abuse and dependence with opioid use disorder, which is diagnosed when a patient meets at least 2 of the 11 revised diagnostic criteria.
Recent amendments to the Drug Addiction Treatment Act of 2000 have increased the patient limit to 275 for physicians who have had a waiver to treat 100 patients with buprenorphine for at least one year and who meet additional criteria.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Combined buprenorphine/naloxone medications are preferred over buprenorphine monotherapy because of their lower abuse potential, unless a patient is pregnant, lactating, or allergic to naloxone. | C | 17, 19 |
| Patient selection is one key to successful buprenorphine medication-assisted therapy in the primary care setting. | C | 13, 17, 19 |
| To reduce the risk of precipitated withdrawal, opioid-dependent patients should wait until they are experiencing mild to moderate withdrawal before taking the first dose of buprenorphine. | C | 13, 15, 17, 19 |
| Validated clinical scales that measure withdrawal symptoms may be used to assist in the evaluation of patients with opioid use disorder. | C | 17–19 |
| Clonidine may be used at dosages of 0.1 to 0.3 mg every 6 to 8 hours to assist in the treatment of opioid use disorder. | C | 17, 19, 20 |
Diagnostic Criteria for Opioid Use Disorder
The Diagnostic and Statistical Manual of Mental Disorders, 5th ed., substantially changed the diagnostic criteria and terminology for substance use disorders.7 The term opioid use disorder replaced the older terms opioid abuse and dependence. Opioid use disorder exists on a spectrum from mild to severe. The diagnosis is made when a patient meets at least two of the 11 revised diagnostic criteria (Table 1).7 However, tolerance is not considered a diagnostic criterion if the patient is taking opioids under medical supervision.7

|
| Specify if: |
| In early remission: After full criteria for opioid use disorder were previously met, none of the criteria for opioid use disorder have been met for at least 3 months but for less than 12 months (with the exception that Criterion 4A, “Craving, or a strong desire or urge to use opioids,” may be met). |
| In sustained remission: After full criteria for opioid use disorder were previously met, none of the criteria for opioid use disorder have been met at any time during a period of 12 months or longer (with the exception that Criterion 4A, “Craving, or a strong desire or urge to use opioids,” may be met). |
| Specify if: |
| On maintenance therapy: This additional specifier is used if the individual is taking a prescribed antagonist medication such as methadone or buprenorphine and none of the criteria for opioid use disorder have been met for that class of medication (except tolerance to or withdrawal from the agonist). This category also applies to those individuals being maintained on a partial agonist, an agonist/antagonist, or a full antagonist such as oral naltrexone or depot naltrexone. |
| In a controlled environment: This additional specifier is used if the individual is in an environment where access to opioids is restricted. |
| Specify current severity: |
| Mild: Presence of 2–3 symptoms. |
| Moderate: Presence of 4–5 symptoms. |
| Severe: Presence of 6 or more symptoms. |
Pathophysiology
The pathophysiology of opioid use disorder largely involves activation of the μ opioid receptor in the mesolimbic or reward pathway of the mid- and forebrain, which in turn activates the release of dopamine, which produces euphoria and pain relief (see Figure 1 at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2994593/figure/F1/). The reward system activates regions of the brain that regulate movement, emotion, motivation, and feelings of pleasure. When activated by normal behaviors, this system functions as the brain's “pleasure center.” Overstimulating the system with opioids causes euphoric effects, which strongly reinforce the behavior of drug use.8 In persons who develop tolerance to opioids, higher doses are required to achieve the same amount of dopamine release. Whereas norepinephrine production is reduced with limited opioid use, it normalizes with prolonged use, and is then poorly regulated in the absence of opioids, leading to withdrawal symptoms.9
Addiction is a chronic brain disease with onset predominantly during adolescence that affects motivation, inhibition, and cognition.10,11 Genetic influences contribute about 50% of the risk of addiction to a substance, and multiple genes are likely involved.12 Shared genetic influences contribute to the risk of opioid use disorder and comorbid psychiatric disorders.13,14 Furthermore, psychological predispositions can influence a person's specific stress response, which may determine if and how that person eventually develops unhealthy patterns of use.
Buprenorphine
PHARMACOLOGY
Buprenorphine is a semisynthetic, highly lipophilic derivative of the opioid alkaloid thebaine. As a partial opioid agonist, its maximal effect is less than that of full agonists, which can limit its effectiveness for pain control, and reaches a ceiling where higher doses do not increase the effect.15,16 This ceiling makes it much safer than full opioid agonists because respiratory depression is limited. Buprenorphine has high affinity for but low intrinsic activity at the μ opioid receptors, and will displace morphine, methadone, and other full opioid agonists from those receptors. Full agonists cannot displace buprenorphine; thus, buprenorphine effectively blunts the high of heroin and other full opioid agonists. Buprenorphine's slow dissociation (disengagement or uncoupling of the drug from the receptor) contributes to its long duration of action.17 Its terminal half-life ranges from three hours after intravenous administration to 28 to 37 hours after sublingual administration.18 The long half-life allows patients to feel stable throughout the day and engage in normal daily activities.
FORMULATIONS
Buprenorphine is a schedule III medication and is manufactured in formulations containing only buprenorphine or combined with naloxone (Table 2). Combined medications are preferred over buprenorphine monotherapy for most patients (except women who are pregnant or lactating and persons allergic to naloxone 19,20) because they are thought to be less likely to be misused.15,17 It is not known whether naloxone is excreted in human milk, and caution should be used when naloxone is administered to a lactating woman. Injecting a combined formulation can precipitate opioid withdrawal because of the high bioavailability of parenteral naloxone.19 In 2016, the U.S. Food and Drug Administration approved the first extended-release buprenorphine subdermal implant for the treatment of opioid use disorder, with effectiveness comparable to an 8-mg daily dosage of buprenorphine.21 These subdermal implants are indicated for maintenance treatment in patients stabilized on low to moderate dosages of transmucosal buprenorphine (8 mg per day) and are designed to provide steady low levels of buprenorphine for six months, reducing the clinical burden and risk of diversion.21
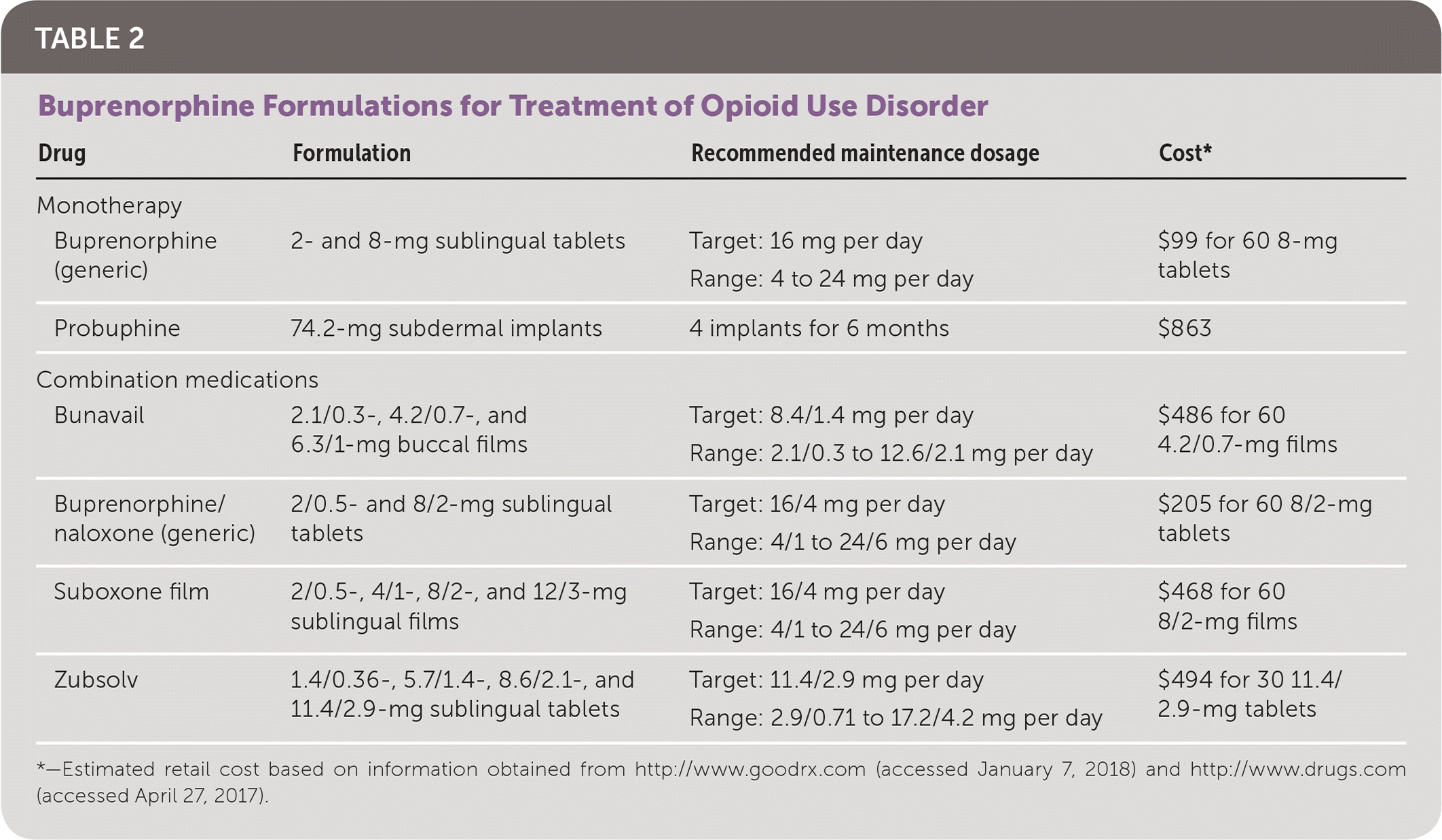
| Drug | Formulation | Recommended maintenance dosage | Cost* | |
|---|---|---|---|---|
| Monotherapy | ||||
| Buprenorphine (generic) | 2- and 8-mg sublingual tablets | Target: 16 mg per day | $99 for 60 8-mg tablets | |
| Range: 4 to 24 mg per day | ||||
| Probuphine | 74.2-mg subdermal implants | 4 implants for 6 months | $863 | |
| Combination medications | ||||
| Bunavail | 2.1/0.3-, 4.2/0.7-, and 6.3/1-mg buccal films | Target: 8.4/1.4 mg per day | $486 for 60 4.2/0.7-mg films | |
| Range: 2.1/0.3 to 12.6/2.1 mg per day | ||||
| Buprenorphine/naloxone (generic) | 2/0.5- and 8/2-mg sublingual tablets | Target: 16/4 mg per day | $205 for 60 8/2-mg tablets | |
| Range: 4/1 to 24/6 mg per day | ||||
| Suboxone film | 2/0.5-, 4/1-, 8/2-, and 12/3-mg sublingual films | Target: 16/4 mg per day | $468 for 60 8/2-mg films | |
| Range: 4/1 to 24/6 mg per day | ||||
| Zubsolv | 1.4/0.36-, 5.7/1.4-, 8.6/2.1-, and 11.4/2.9-mg sublingual tablets | Target: 11.4/2.9 mg per day | $494 for 30 11.4/2.9-mg tablets | |
| Range: 2.9/0.71 to 17.2/4.2 mg per day | ||||
SAFETY
Buprenorphine and combinations of buprenorphine and naloxone are generally well tolerated. Commonly reported adverse effects include anxiety, constipation, dizziness, drowsiness, headache, nausea, and sedation22; serious adverse effects are listed in Table 3.23,24 Buprenorphine's ceiling effect makes it relatively safe in cases of accidental or intentional overdose. However, overdose of buprenorphine combined with other medications may increase morbidity and mortality (Table 4).23,25–27
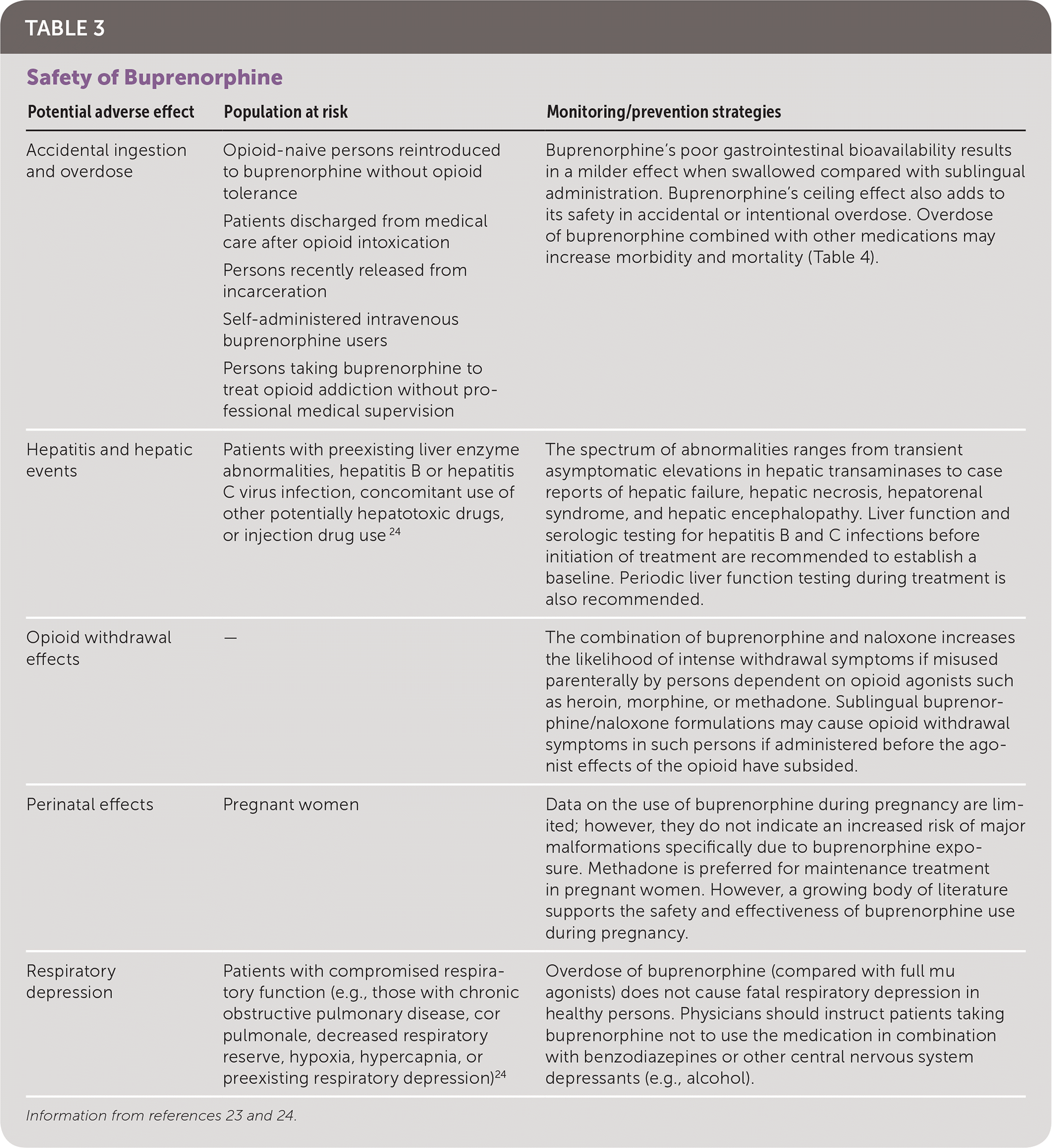
| Potential adverse effect | Population at risk | Monitoring/prevention strategies |
|---|---|---|
| Accidental ingestion and overdose | Opioid-naive persons reintroduced to buprenorphine without opioid tolerance | Buprenorphine's poor gastrointestinal bioavailability results in a milder effect when swallowed compared with sublingualadministration. Buprenorphine's ceiling effect also adds to its safety in accidental or intentional overdose. Overdose of buprenorphine combined with other medications may increase morbidity and mortality (Table 4). |
| Patients discharged from medical care after opioid intoxication | ||
| Persons recently released from incarceration | ||
| Self-administered intravenous buprenorphine users | ||
| Persons taking buprenorphine to treat opioid addiction without professional medical supervision | ||
| Hepatitis and hepatic events | Patients with preexisting liver enzyme abnormalities, hepatitis B or hepatitis C virus infection, concomitant use of other potentially hepatotoxic drugs, or injection drug use 24 | The spectrum of abnormalities ranges from transient asymptomatic elevations in hepatic transaminases to case reports of hepatic failure, hepatic necrosis, hepatorenal syndrome, and hepatic encephalopathy. Liver function and serologic testing for hepatitis B and C infections before initiation of treatment are recommended to establish a baseline. Periodic liver function testing during treatment is also recommended. |
| Opioid withdrawal effects | — | The combination of buprenorphine and naloxone increases the likelihood of intense withdrawal symptoms if misused parenterally by persons dependent on opioid agonists such as heroin, morphine, or methadone. Sublingual buprenorphine/naloxone formulations may cause opioid withdrawal symptoms in such persons if administered before the agonist effects of the opioid have subsided. |
| Perinatal effects | Pregnant women | Data on the use of buprenorphine during pregnancy are limited; however, they do not indicate an increased risk of major malformations specifically due to buprenorphine exposure. Methadone is preferred for maintenance treatment in pregnant women. However, a growing body of literature supports the safety and effectiveness of buprenorphine use during pregnancy. |
| Respiratory depression | Patients with compromised respiratory function (e.g., those with chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression)24 | Overdose of buprenorphine (compared with full mu agonists) does not cause fatal respiratory depression in healthy persons. Physicians should instruct patients taking buprenorphine not to use the medication in combination with benzodiazepines or other central nervous system depressants (e.g., alcohol). |
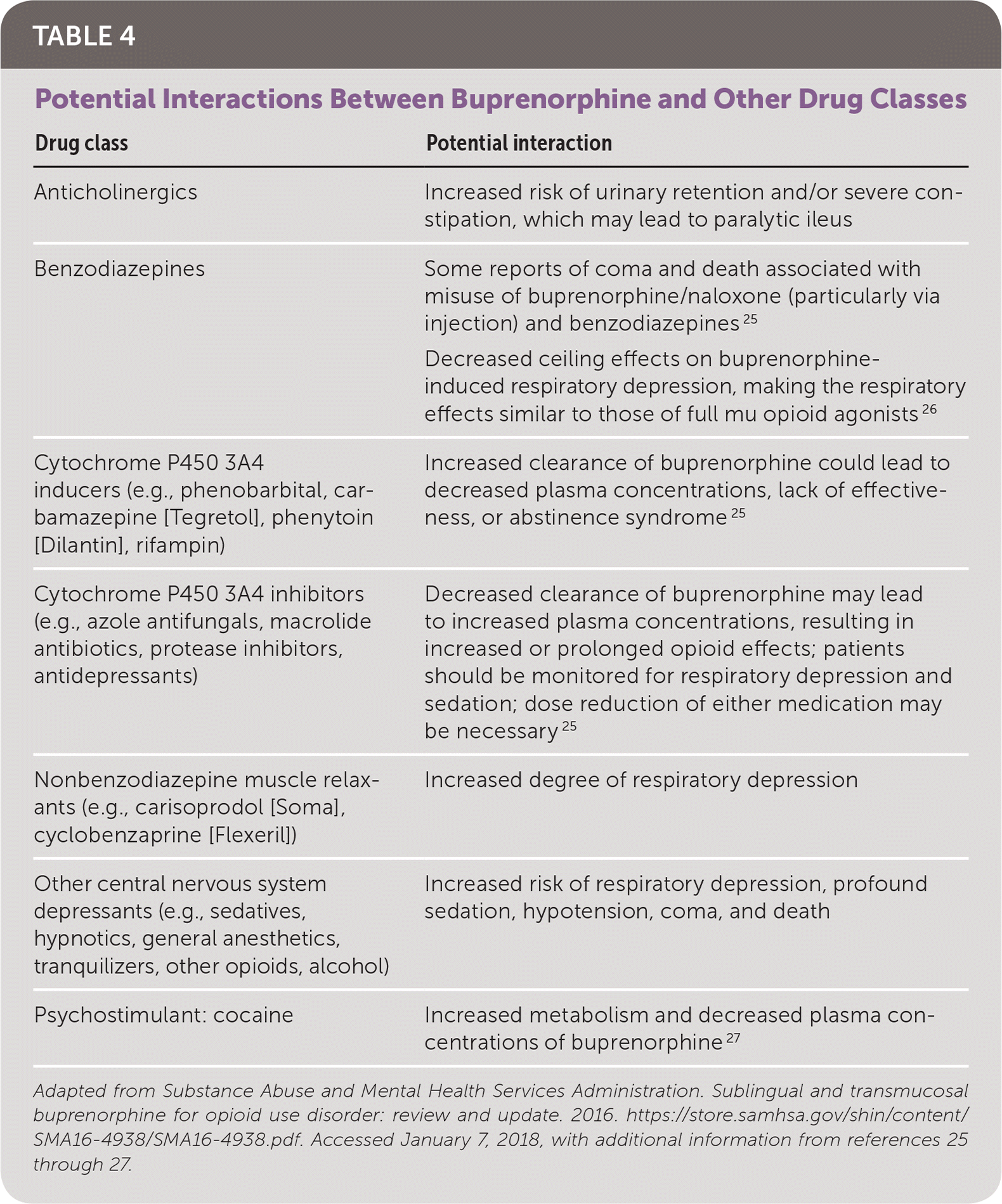
| Drug class | Potential interaction |
|---|---|
| Anticholinergics | Increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus |
| Benzodiazepines | Some reports of coma and death associated with misuse of buprenorphine/naloxone (particularly via injection) and benzodiazepines 25 |
| Decreased ceiling effects on buprenorphine-induced respiratory depression, making the respiratory effects similar to those of full mu opioid agonists 26 | |
| Cytochrome P450 3A4 inducers (e.g., phenobarbital, carbamazepine [Tegretol], phenytoin [Dilantin], rifampin) | Increased clearance of buprenorphine could lead to decreased plasma concentrations, lack of effectiveness, or abstinence syndrome 25 |
| Cytochrome P450 3A4 inhibitors (e.g., azole antifungals, macrolide antibiotics, protease inhibitors, antidepressants) | Decreased clearance of buprenorphine may lead to increased plasma concentrations, resulting in increased or prolonged opioid effects; patients should be monitored for respiratory depression and sedation; dose reduction of either medication may be necessary 25 |
| Nonbenzodiazepine muscle relaxants (e.g., carisoprodol [Soma], cyclobenzaprine [Flexeril]) | Increased degree of respiratory depression |
| Other central nervous system depressants (e.g., sedatives, hypnotics, general anesthetics, tranquilizers, other opioids, alcohol) | Increased risk of respiratory depression, profound sedation, hypotension, coma, and death |
| Psychostimulant: cocaine | Increased metabolism and decreased plasma concentrations of buprenorphine 27 |
Drug Addiction Treatment Act of 2000
The Drug Addiction Treatment Act of 2000 (DATA 2000) expanded access to treatment for opioid use disorder by legalizing office-based maintenance therapy outside of licensed opioid treatment programs. It allows physicians who meet certain qualifications to treat opioid use disorder with schedule III, IV, and V narcotic medications that have been approved for that indication. Buprenorphine is the only narcotic besides methadone currently approved for treatment of opioid use disorder.
To obtain a DATA 2000 waiver, physicians must notify the Substance Abuse and Mental Health Services Administration of their intent to begin dispensing and/or prescribing this treatment, have a current state medical license and a valid Drug Enforcement Administration registration number, and complete eight hours of required training 11 (eTable A). A DATA 2000 waiver typically enables physicians to treat up to 30 patients at any given time during the first year, then up to 100 patients in subsequent years. Recent amendments to the law have increased the patient limit to 275 for physicians who have had their waiver to treat 100 patients for at least one year and who meet several additional criteria. Authorized nurse practitioners and physician assistants may obtain waivers to treat up to 30 patients after completing 24 hours of training and can also request to have their limit increased to 100 patients after one year.28
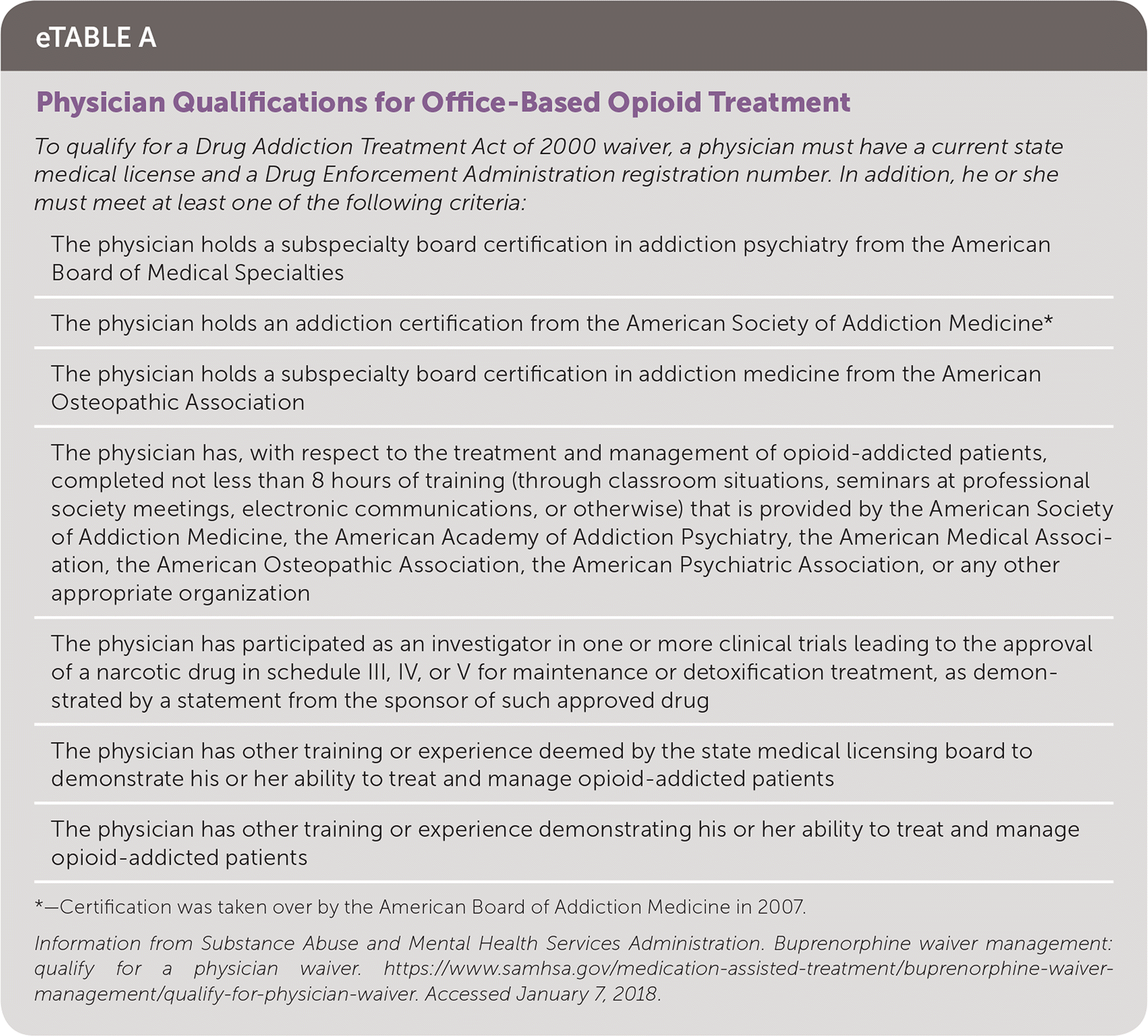
| To qualify for a Drug Addiction Treatment Act of 2000 waiver, a physician must have a current state medical license and a Drug Enforcement Administration registration number. In addition, he or she must meet at least one of the following criteria: |
| The physician holds a subspecialty board certification in addiction psychiatry from the American Board of Medical Specialties |
| The physician holds an addiction certification from the American Society of Addiction Medicine* |
| The physician holds a subspecialty board certification in addiction medicine from the American Osteopathic Association |
| The physician has, with respect to the treatment and management of opioid-addicted patients, completed not less than 8 hours of training (through classroom situations, seminars at professional society meetings, electronic communications, or otherwise) that is provided by the American Society of Addiction Medicine, the American Academy of Addiction Psychiatry, the American Medical Association, the American Osteopathic Association, the American Psychiatric Association, or any other appropriate organization |
| The physician has participated as an investigator in one or more clinical trials leading to the approval of a narcotic drug in schedule III, IV, or V for maintenance or detoxification treatment, as demonstrated by a statement from the sponsor of such approved drug |
| The physician has other training or experience deemed by the state medical licensing board to demonstrate his or her ability to treat and manage opioid-addicted patients |
| The physician has other training or experience demonstrating his or her ability to treat and manage opioid-addicted patients |
Prescribing Buprenorphine for Opioid Use Disorder
Patients with opioid use disorder who are in early recovery and are not using full agonist (methadone) or partial agonist (buprenorphine) MAT are at increased risk of opioid overdose due to decreased tolerance.29 Buprenorphine MAT is as effective as methadone in terms of treatment retention and decreased opioid use when prescribed at fixed dosages of at least 7 mg per day; dosages of 16 mg per day are clearly superior to placebo.30
Patient selection is one key to successful buprenorphine MAT in the primary care setting.13,17,19 Characteristics associated with successful treatment include stable or controlled medical or psychiatric comorbidities and a safe, substance-free environment.31 Patients with active comorbid benzodiazepine or other sedative use disorders are at increased risk of adverse events.31 Patients with unstable living environments can have challenges with safeguarding their medications and prioritizing MAT engagement while meeting acute needs (food and shelter); these patients may be better served in a specialty care setting.
INDUCTION
Baseline urine drug testing, informed consent, and review of the treatment contract should be completed before buprenorphine induction. To reduce the risk of precipitated withdrawal, the patient is typically instructed to arrive at the office in mild to moderate withdrawal (i.e., having last used an opioid at least eight to 12 hours before their appointment).13,15,17,19 A validated clinical scale, such as the Clinical Opiate Withdrawal Scale, may be used to document the patient's symptoms and their resolution after buprenorphine dosing.32 Patients who are not exhibiting active withdrawal symptoms at their induction appointment should be rescheduled and reminded of the need to initiate therapy when they are in mild to moderate withdrawal. Alternatively, induction can be done at home with early follow-up in the office, especially for patients with prior MAT experience and a physician experienced in prescribing MAT.
At induction, patients are typically monitored in the office at 60-minute intervals after the first dose of buprenorphine, with continued dose titration until withdrawal symptoms abate. Close follow-up—typically the next day, but no later than one week—is appropriate.33
Clonidine (0.1 mg every six to eight hours, increasing weekly in 0.1-mg increments to 0.3 mg every six to eight hours) is a useful adjunct to buprenorphine MAT, increasing abstinence rates and decreasing stress-related cravings.17,19,20,34 Although the risk of buprenorphine overdose is low in opioid-dependent patients, consideration should be given to coprescribing a naloxone overdose kit to patients receiving buprenorphine MAT. More information on prescribing naloxone is available.
Patients with opioid use disorder should be screened at the initial visit or during early follow-up for common comorbidities, including human immunodeficiency virus infection, viral hepatitis, syphilis, tuberculosis, and cervical dysplasia. Hepatitis A and B vaccination should be offered to patients who are not immune.31
FOLLOW UP
Once a patient reaches a stable dose of buprenorphine (i.e., a dose at which opioid use stops), withdrawal signs and symptoms abate, and cravings are relieved or minimized, follow-up intervals can be lengthened from weekly to biweekly or monthly. Sporadic opioid use is not uncommon in the first few months of MAT and should be addressed by increased visit frequency and more intensive engagement with behavioral therapies (e.g., cognitive behavior therapy, contingency management, motivational enhancement). Case management may also be helpful. If a patient is not able to abstain from opioid use despite buprenorphine dosages sufficient to saturate opioid receptors (typically 24 mg per day) and sustained engagement in behavioral therapies, referral for full agonist MAT with methadone is indicated.33
Follow-up visits should include documentation of relapses; reemergence of cravings or withdrawal; pill or wrapper counts; checks of state prescription drug database records; and random urine test results for illicit drugs, prescription opioids, hypnotic sedatives (benzodiazepines and barbiturates), and buprenorphine and its metabolites.33 Additionally, patient requests for dose increases or decreases should be noted, as should any adverse effects. The most common adverse effect of buprenorphine MAT is constipation, which can be managed with over-the-counter or prescription stool softeners or laxatives. Patients should have access to counseling as well.
DISCONTINUATION
Buprenorphine MAT is not a time-limited treatment. Continued abstinence and recovery are strongly linked to continued MAT.25 Discontinuation of buprenorphine MAT is most often due to patient preference, change in the patient's access to treatment, change in insurance status, or diversion. When buprenorphine MAT is discontinued, a slow taper is preferred unless the discontinuation is for medication diversion.
The Providers' Clinical Support System for Medication Assisted Treatment is a free, evidence-based resource for further information and training on prescribing buprenorphine MAT in the primary care setting.
Editor's Note: The AAFP's position paper, Chronic Pain Management and Opioid Misuse: A Public Health Concern, serves as a call to action for family physicians, with recommendations and resources for treating pain and managing medication misuse/abuse, including medication-assisted treatment. The AAFP also has a Chronic Pain Management Tooklit.
This article updates a previous article on this topic by Donaher and Welsh.35
Data Sources: A comprehensive English-language literature review of several databases was conducted, including Essential Evidence Plus, the Cochrane Database of Systematic Reviews, clinical decision rules, the Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, the American Society of Addiction Medicine, the Drug Enforcement Administration, the U.S. Food and Drug Administration, and a focused PubMed search. Keywords included buprenorphine, opioid use disorders, and medication-assisted treatment. Search dates: April 25, 2017, through January 20, 2018.
