
Am Fam Physician. 2018;97(8):online
Related Putting Prevention into Practice: Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women.
Summary of Recommendations and Evidence
The USPSTF recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women (Table 1). D recommendation.
The USPSTF recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal women who have had a hysterectomy. D recommendation.

| Population | Postmenopausal women | Postmenopausal women who have had a hysterectomy |
| Recommendation | Do not use combined estrogen and progestin for the primary prevention of chronic conditions. Grade: D | Do not use estrogen alone for the primary prevention of chronic conditions. Grade: D |
| This recommendation statement applies to postmenopausal women who are considering hormone therapy for the primary prevention of chronic medical conditions. It does not apply to women who are considering hormone therapy for the management of menopausal symptoms, or to women who have had premature menopause (primary ovarian insufficiency) or surgical menopause. | ||
| Risk assessment | These recommendations apply to an average-risk population. Risk factors for a specific chronic condition or individual characteristics that affect the likelihood of experiencing a specific therapy-associated adverse event may cause a woman's net balance of benefits and harms to differ from that of the average population. | |
| Preventive medication | Hormone therapy refers to the use of combined estrogen and progestin in women with an intact uterus, or estrogen alone in women who have had a hysterectomy, taken at or after the time of menopause. For this recommendation, the USPSTF considered evidence on systemic (i.e., oral or transdermal) menopausal hormone therapy but not local formulations (i.e., creams or rings), since they are not generally used for primary prevention. Several different formulations of menopausal hormone therapy are approved by the U.S. Food and Drug Administration for use in the United States; the specific formulation used in the Women's Health Initiative, the largest trial, was 0.625 mg/day of oral conjugated equine estrogens, with or without 2.5 mg/day of medroxyprogesterone acetate. | |
| Other relevant USPSTF recommendations | The USPSTF recommends behavioral counseling interventions to promote a healthful diet and physical activity for the prevention of cardiovascular disease in women who are overweight or obese and have additional cardiovascular disease risk factors. The USPSTF recommends daily low-dose aspirin use to decrease the risk of colorectal cancer and cardiovascular disease in appropriate candidates. The USPSTF recommends offering medications such as tamoxifen and raloxifene to women at increased risk of breast cancer who do not have contraindications and are at low risk of adverse medication effects to decrease the risk of breast cancer. | |
Rationale
IMPORTANCE
Menopause is defined as the permanent cessation of a woman's menstrual cycle. It is typically defined in retrospect, 12 months after a woman's final menstrual period. Menopause occurs at a median age of 51.3 years, and the average U.S. woman who reaches menopause is expected to live another 30 years. The prevalence and incidence of most chronic conditions, such as coronary heart disease, dementia, stroke, fractures, and breast cancer, increase with age; however, the excess risk for these conditions that can be attributed to menopause alone is uncertain. Since the publication of findings from the Women's Health Initiative (WHI) that hormone therapy use was associated with serious adverse health effects in postmenopausal women, use of menopausal hormone therapy has declined, from 44% of U.S. women using or having used hormone therapy in 1988–1994 to 4.7% of women in 2010.1
BENEFITS OF PREVENTIVE MEDICATION
Combined Estrogen and Progestin. Many health outcomes potentially associated with the use of hormone therapy in postmenopausal women have been examined. The USPSTF found convincing evidence that use of combined estrogen and progestin has a moderate benefit in reducing the risk of fractures in postmenopausal women and adequate evidence that it has a small benefit in reducing the risk of diabetes.
Estrogen Alone. The use of estrogen without progestin has generally been restricted to women who have had a hysterectomy, because unopposed estrogen use increases the risk of endometrial cancer in women with an intact uterus. The USPSTF found convincing evidence that use of estrogen alone has a moderate benefit in reducing the incidence of fractures in postmenopausal women. The USPSTF found adequate evidence that the use of estrogen alone has a moderate benefit in reducing the risk of developing or dying of invasive breast cancer and a small benefit in reducing the risk of diabetes. The USPSTF found convincing evidence that estrogen use does not have a beneficial effect on risk of coronary heart disease.
HARMS OF PREVENTIVE MEDICATION
Combined Estrogen and Progestin. The USPSTF found convincing evidence that use of combined estrogen and progestin is associated with moderate harms, including increased risk of invasive breast cancer and venous thromboembolism, and a small to moderate harm of increased risk of coronary heart disease. The USPSTF also found adequate evidence of other moderate harms, such as increased risk of stroke, dementia, gallbladder disease, and urinary incontinence.
Estrogen Alone. The USPSTF found adequate evidence that use of estrogen alone is associated with moderate harms, including increased risk of stroke, dementia, gallbladder disease, urinary incontinence, and venous thromboembolism.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that the use of combined estrogen and progestin has no net benefit for the primary prevention of chronic conditions in most postmenopausal women with an intact uterus.
The USPSTF concludes with moderate certainty that the use of estrogen alone has no net benefit for the primary prevention of chronic conditions in most postmenopausal women who have had a hysterectomy.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation statement applies to asymptomatic, postmenopausal women who are considering hormone therapy for the primary prevention of chronic medical conditions. It does not apply to women who are considering hormone therapy for the management of menopausal symptoms, such as hot flashes or vaginal dryness. It also does not apply to women who have had premature menopause (primary ovarian insufficiency) or surgical menopause.
ASSESSMENT OF RISK
This recommendation statement applies to an average-risk population. Risk factors for a specific chronic condition or individual characteristics that affect the likelihood of experiencing a specific therapy-associated adverse event may cause a woman's net balance of benefits and harms to differ from that of the average population.
TREATMENT AND INTERVENTION
Menopausal hormone therapy refers to the use of combined estrogen and progestin in women with an intact uterus, or estrogen alone in women who have had a hysterectomy, taken at or after the time of menopause. For this recommendation, the USPSTF considered evidence on the benefits and harms of systemic (i.e., oral or transdermal) menopausal hormone therapy but not local formulations (e.g., creams or rings) of hormone therapy, because these are not generally used for the primary prevention of chronic conditions.
Indications for hormone therapy approved by the U.S. Food and Drug Administration (FDA) in menopausal women are limited to the treatment of menopausal symptoms and the prevention of postmenopausal osteoporosis. An FDA-issued black box warning indicates that estrogen therapy, with or without progestin, should be prescribed at the lowest effective dose and for the shortest duration consistent with the patient's treatment goals and risks.2
Several different formulations of menopausal hormone therapy are approved by the FDA for use in the United States; the specific formulation used in the WHI trial, the largest trial reviewed by the USPSTF, was 0.625 mg/day of oral conjugated equine estrogens, with or without 2.5 mg/day of medroxyprogesterone acetate. Currently, evidence to determine whether different types, doses, or modes of delivery of hormone therapy affect its benefit-to-harm profile for the prevention of chronic conditions is limited.1
The use of menopausal hormone therapy is associated with both benefits and harms. Combined estrogen and progestin use is associated with a decreased risk of fractures, diabetes, and colorectal cancer; however, it is also associated with an increased risk of invasive breast cancer, coronary heart disease, thromboembolic events, stroke, dementia, gallbladder disease, and self-reported urinary incontinence. Estrogen use alone is associated with a decreased risk of fractures, invasive breast cancer, and diabetes; however, it is also associated with an increased risk of thromboembolic events, stroke, dementia, gall-bladder disease, and self-reported urinary incontinence. The reason for the discordant effect of estrogen alone compared with combined estrogen and progestin on the risk of invasive breast cancer is unclear. Table 2 and Table 3 show the estimated absolute event rate differences associated with the use of combined estrogen and progestin and estrogen alone, compared with placebo, for these health outcomes.1
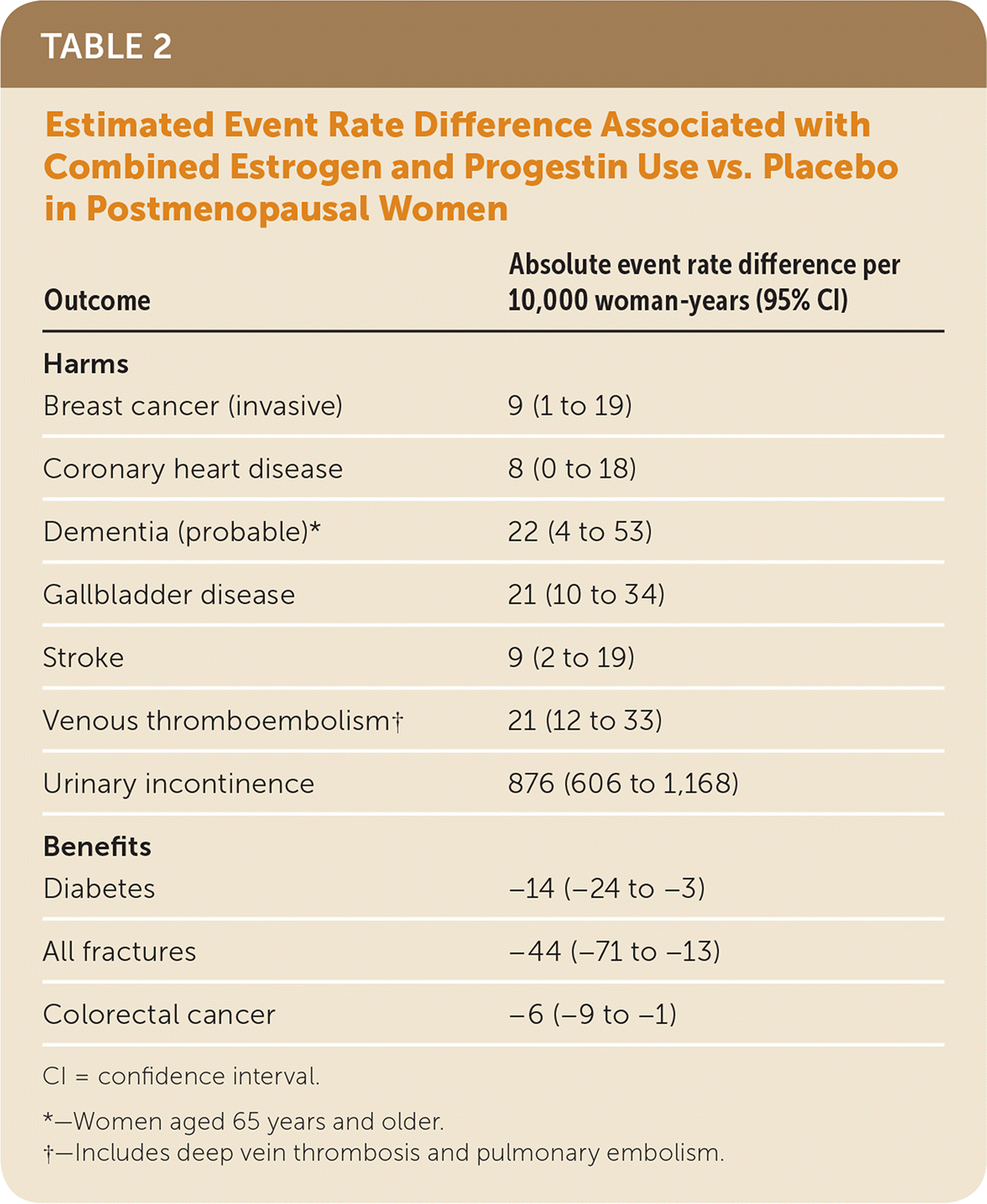
| Outcome | Absolute event rate difference per 10,000 woman-years (95% CI) |
|---|---|
| Harms | |
| Breast cancer (invasive) | 9 (1 to 19) |
| Coronary heart disease | 8 (0 to 18) |
| Dementia (probable)* | 22 (4 to 53) |
| Gallbladder disease | 21 (10 to 34) |
| Stroke | 9 (2 to 19) |
| Venous thromboembolism† | 21 (12 to 33) |
| Urinary incontinence | 876 (606 to 1,168) |
| Benefits | |
| Diabetes | −14 (−24 to −3) |
| All fractures | −44 (−71 to −13) |
| Colorectal cancer | −6 (−9 to −1) |
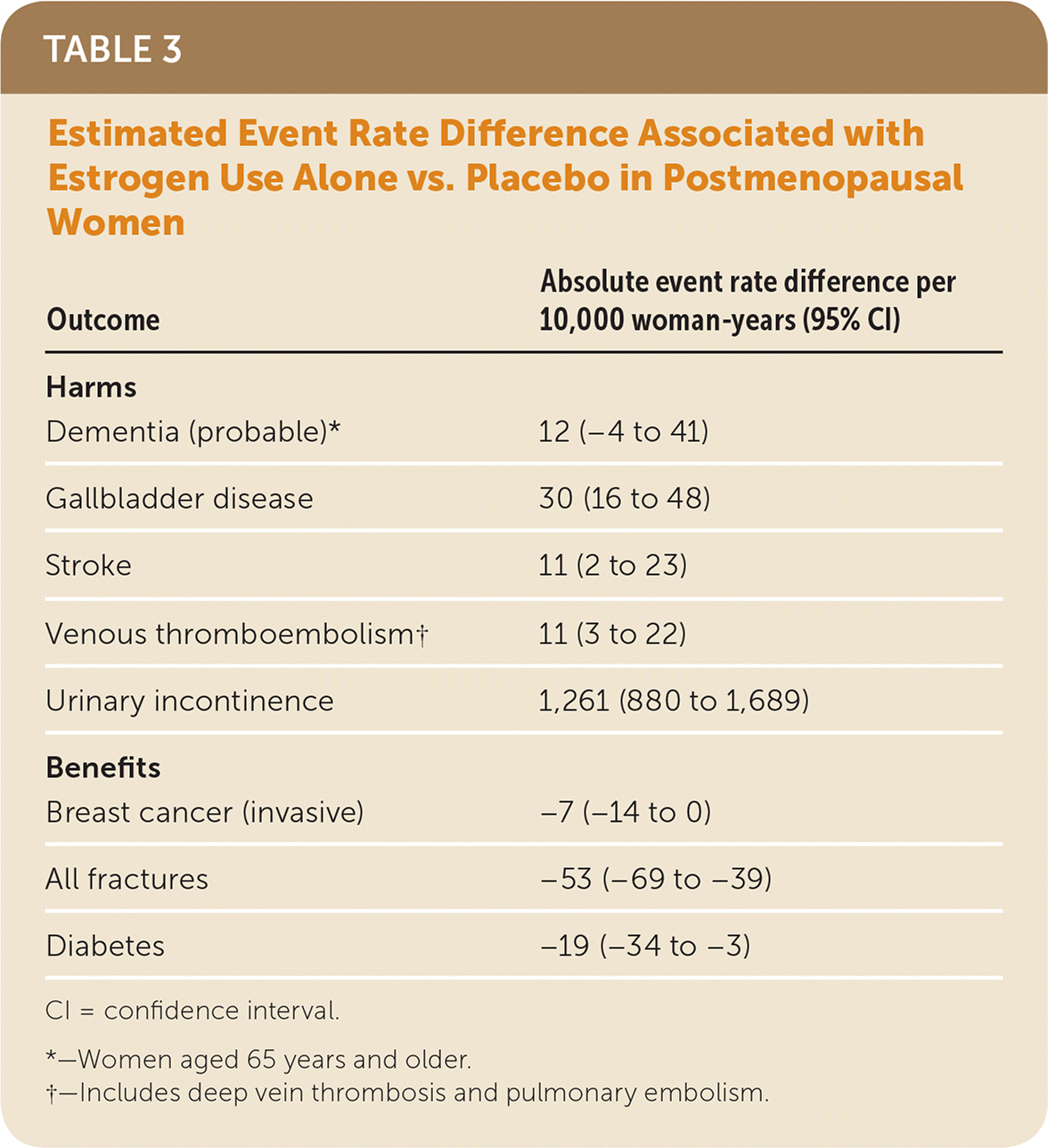
| Outcome | Absolute event rate difference per 10,000 woman-years (95% CI) |
|---|---|
| Harms | |
| Dementia (probable)* | 12 (−4 to 41) |
| Gallbladder disease | 30 (16 to 48) |
| Stroke | 11 (2 to 23) |
| Venous thromboembolism† | 11 (3 to 22) |
| Urinary incontinence | 1,261 (880 to 1,689) |
| Benefits | |
| Breast cancer (invasive) | −7 (−14 to 0) |
| All fractures | −53 (−69 to −39) |
| Diabetes | −19 (−34 to −3) |
OTHER APPROACHES TO PREVENTION
Several interventions and preventive medications to reduce the risk of chronic conditions in women have been studied. For example, the use of medications such as tamoxifen and raloxifene in women at increased risk of breast cancer who do not have contraindications and are at low risk of adverse medication effects is a potential strategy to reduce risk of breast cancer.3 The USPSTF recommends behavioral counseling interventions to promote a healthful diet and physical activity for the prevention of cardiovascular disease in adults who are overweight or obese and have additional cardiovascular disease risk factors.4 The USPSTF also recommends daily use of low-dose aspirin to decrease the risk of colorectal cancer and cardiovascular disease in appropriate candidates.5
This recommendation statement was first published in JAMA. 2017;318(22):2224–2233.
The “Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
