
Am Fam Physician. 2018;97(8):538-539
Author disclosure: No relevant financial affiliations.

Details for This Review
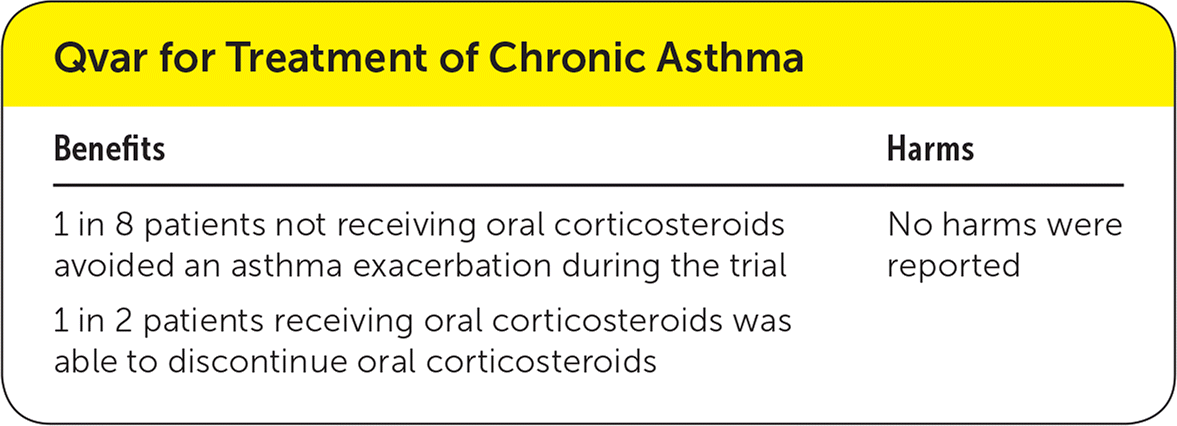
| Benefits | Harms |
|---|---|
| 1 in 8 patients not receiving oral corticosteroids avoided an asthma exacerbation during the trial | No harms were reported |
| 1 in 2 patients receiving oral corticosteroids was able to discontinue oral corticosteroids |
Study Population: Children and adults with a clinical diagnosis of chronic asthma
Efficacy End Points: Asthma exacerbation causing withdrawal from the trial; discontinuation of oral corticosteroids
Harm End Points: Oropharyngeal adverse effects such as dysphonia and oral candidiasis
Narrative: During an asthma attack there is airway narrowing, wheezing, chest tightness, and coughing. Inhaled corticosteroids are used to improve pulmonary function and reduce the frequency of asthma attacks without exposing the patients to the adverse effects of systemic corticosteroids. Inhaled corticosteroids are the standard of care in preventing asthma attacks. Qvar is a new formulation of inhaled beclomethasone using a hydrofluoroalkane-134a propellant, in which the medicine is delivered in a solution. The smaller particle sizes in the hydrofluoroalkane-134a allows the medication to travel into the smaller parts of the lungs.
The trials included in a Cochrane review studied the effectiveness of Qvar in two sets of patients with chronic asthma: those receiving oral corticosteroids and those not receiving them. These trials used different doses and delivery devices. In our review, we present only the combined data provided by the Cochrane review (any dose, any device).1
Other inhaled corticosteroids available are fluticasone (Flovent) and budesonide (Pulmicort). A different Cochrane review compared the effectiveness of three inhaled corticosteroids (fluticasone, beclomethasone, and budesonide) for treating patients with chronic asthma.2 Fluticasone at one-half the daily dosage of beclomethasone or budesonide was at least as effective as the other two medications in improving airway opening. There were not enough data to compare the effectiveness of the three formulations in preventing acute asthma exacerbations. When given in the same dosage as the other two, fluticasone was associated with increased hoarseness, although it did not increase the risk of other adverse effects associated with corticosteroids such as oral thrush or sore throat.
In patients not receiving oral corticosteroids, the use of Qvar was associated with a lower risk of withdrawal from the trial because of asthma exacerbation (absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 9% to 16%; number needed to treat [NNT] = 8). Similarly, the use of Qvar allowed more patients to discontinue their oral corticosteroid (ARR = 46%; 95% CI, 35% to 56%; NNT = 2).
The risk of oral candidiasis and the overall oropharyngeal adverse effects were not statistically different between the groups in patients receiving oral corticosteroids.
Caveats: The source Cochrane review included two sets of populations among patients with chronic asthma, one group receiving oral corticosteroids and the other group not receiving them. The Cochrane review also measures the efficacy end points for various doses, delivery devices, and disease severities. For simplicity, we present only the data for any dose of Qvar irrespective of disease severity or the delivery device type.
Most of the trials included in the review did not measure admission rates as an outcome. Most trials measured surrogate outcomes such as forced expiratory volume in one second, reduction in the frequency of use of rescue beta2 agonist, and the frequency of use of oral corticosteroids. These outcomes would only be meaningful if they could be translated into patient-centered outcomes such as reduction in emergency department visits or hospital admissions.
There was notable heterogeneity between the studies because of the different doses, disease severities, and delivery methods that were studied. Because of these differences, it is hard to combine the data for a singular conclusion.
It must be noted that the latest guidelines published by the National Heart, Lung, and Blood Institute also recommend Qvar for patients with chronic asthma.3
Copyright 2018 The NNT Group (theNNT.com). Used with permission.
This review is available from the NNT Group at http://www.thennt.com/nnt/qvar-treatment-chronic-asthma/.
