
This is an updated version of the article that appeared in print. See Editor's Note.
Am Fam Physician. 2018;97(10):668-670
Author disclosure: No relevant financial affiliations.
Implantable buprenorphine (Probuphine) is an implantable partial opioid agonist. It is labeled for the treatment of patients with opioid use disorder who have achieved and sustained clinical stability on a transmucosal buprenorphine product at low to moderate dosages, defined as no more than 8 mg per day.1
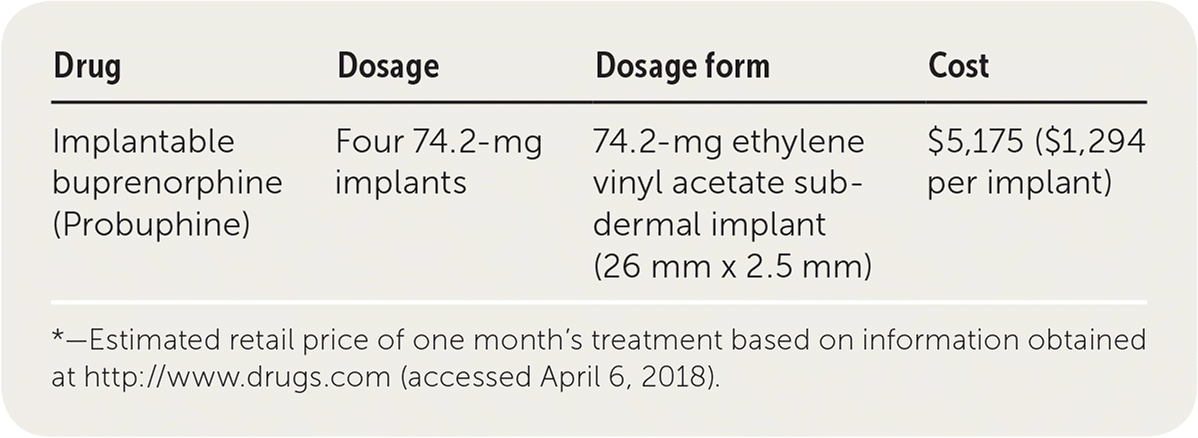
| Drug | Dosage | Dosage form | Cost |
|---|---|---|---|
| Implantable buprenorphine (Probuphine) | Four 74.2-mg implants | 74.2-mg ethylene vinyl acetate subdermal implant (26 mm × 2.5 mm) | $5,175 ($1,294 per implant) |
Safety
Serious complications are related to improper insertion and removal of the buprenorphine implant, as well as improper initiation in patients taking other types of opioid therapy who are not already stabilized on a buprenorphine regimen. The product can be prescribed, inserted, and removed only by health care professionals who have received certification and training through the U.S. Food and Drug Administration's Probuphine Risk Evaluation and Mitigation Strategy program. Opioid withdrawal is precipitated by protrusion or expulsion of implantable buprenorphine, purposeful removal of the device, or initiation of implantable buprenorphine in patients dependent on full opioid agonists (i.e., heroin, morphine, and methadone).1
Other forms of buprenorphine are used to treat opioid use disorder in pregnant women; however, implantable buprenorphine should not be used in pregnant patients because of the inflexibility of dosing. Patients who become pregnant while receiving treatment may require augmented therapy with an additional buprenorphine product to control symptoms. Newborns of mothers treated with implantable buprenorphine may be at increased risk of neonatal opioid withdrawal syndrome and respiratory depression, as with other opioid treatments. Buprenorphine is present in low levels in breast milk and is considered compatible with breastfeeding.1 This medication has not been evaluated for teratogenicity in humans, but birth defects have not been shown to increase in animals given dosages equivalent to the recommended dosage of 8 to 16 mg per day.1
Tolerability
Implantable buprenorphine is well tolerated by patients. In clinical studies of buprenorphine and placebo implants, there were similar rates of discontinuation (5.6% vs. 6.9%) due to adverse effects.2 The most commonly reported adverse effects include pain, pruritus, and erythema at the implant site, affecting approximately 13% of patients. Headache has been reported by approximately 5% of patients.2
Effectiveness
Implantable buprenorphine is effective in stable patients with previously diagnosed opioid use disorder compared with patients taking 8 mg per day of transmucosal buprenorphine.3 In a single study of 176 patients with opioid use disorder on a stable maintenance dosage (8 mg or 16 mg per day) of buprenorphine, those who switched to the buprenorphine implant did not experience an increase in observed withdrawal symptoms or relapse events compared with continued treatment with transmucosal buprenorphine.4 Implantable buprenorphine has not been shown to be effective for patients beginning treatment for opioid use disorder.2 It has not been studied longer than 12 months or in patients on unstable opioid dosages, newly diagnosed patients, pregnant women, or patients displaying uncontrolled psychiatric illness. Additionally, the implants have a safeguard to prevent drug diversion secondary to the matrix in which the active drug is stored. Implantable buprenorphine has not been evaluated as an alternative to other treatments such as naltrexone (Vivitrol) or methadone.
Price
Four buprenorphine implants cost approximately $5,175 (about $1,294 per implant). This is in addition to the cost of inserting the implant. Implantable buprenorphine is considerably more expensive than transmucosal buprenorphine, which costs about $60 for a one-month supply, but it is comparable to injectable naltrexone, which costs approximately $1,300 per injection. Probuphine implantable devices are likely to be covered by most insurers; however, it is unclear whether the devices will be reimbursable by all third-party plans.
Simplicity
Implantable buprenorphine requires a prescription from a physician and is generally not available at a pharmacy. Only clinicians with a medication-assisted opioid-dependency treatment waiver (Drug Abuse Treatment Act of 2000) and who have further training and certification can prescribe and implant buprenorphine. Once implanted, buprenorphine is released for six months, after which the implants can be removed and new implants may be placed in the opposite arm. Implants should not be removed by anyone who has not received training. Patients receiving treatment may require additional transmucosal doses of buprenorphine, and a patient in acute pain may require much higher doses of a full opioid agonist to overcome the antagonistic effects of buprenorphine.1
Bottom Line
Implantable buprenorphine is an expensive alternative for patients on a stable dosage of transmucosal buprenorphine. Based on current research and guidance, implantable buprenorphine should not be used as initial treatment for opioid use disorder or as a substitute for medication-assisted treatments other than transmucosal buprenorphine.2 Its long duration of action makes it more convenient for patients, although one limitation of the implant is that it removes the need for return office visits for monitoring and counseling that can be enforced with limited-duration prescriptions of other medication-assisted treatment.
Editor's Note: Although the product label approved by the U.S. Food and Drug Administration uses the old terminology "opioid dependence," we have chosen to use the more accurate Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnosis "opioid use disorder" (OUD) because physiologic dependence on opioids alone is not an indication for medication-assisted therapy for OUD.
