
Am Fam Physician. 2018;97(11):online
Author disclosure: No relevant financial affiliations.

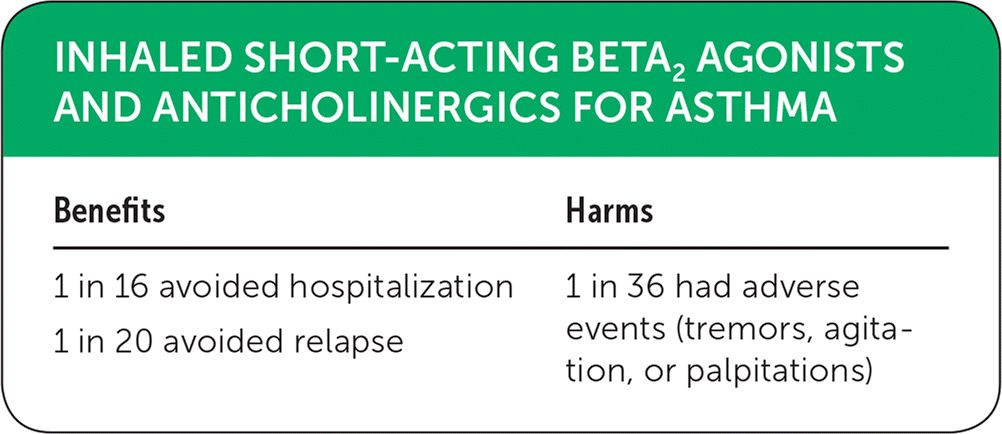
| Benefits | Harms |
|---|---|
| 1 in 16 avoided hospitalization | 1 in 36 had adverse events (tremors, agitation, or palpitations) |
| 1 in 20 avoided relapse |
Details for This Review
Study Population: Patients 16 years or older with asthma who presented to the emergency department (ED) with an asthma exacerbation
Efficacy End Points: Reduction in hospitalization, reduction in return visits to the ED
Harm End Points: Increase in adverse events such as tremors, agitation, and palpitations
Narrative: In the United States, 9% of patients who present with an asthma exacerbation require hospitalization.1 The direct costs for asthma treatment in the United States, including prescriptions, hospitalizations, and clinic and ED visits, total approximately $5.1 billion annually.2 For years, the mainstay of treatment for acute asthma exacerbations has been short-acting beta2 agonists (SABAs), such as albuterol. Albuterol works primarily to promote bronchodilation. Additionally, short-acting anticholinergics, such as ipratropium (Atrovent), work to reduce bronchorrhea and have a milder impact on bronchodilation than SABAs. The combination of SABAs and short-acting anticholinergics has been studied and suggests a possible synergistic effect that lengthens symptom management and reduces the need for inpatient stays.
This Cochrane review included 23 controlled or randomized trials with a total of 2,724 patients.3 Sixteen of these trials (2,120 participants) found that those receiving combined short-acting anticholinergic and SABA therapy had a lower rate of hospitalization than those receiving a SABA alone (relative risk [RR] = 0.72; 95% confidence interval [CI], 0.59 to 0.87). The estimated 65 fewer patients per 1,000 admitted to the hospital (166 vs. 231, respectively) translates to a number needed to treat (NNT) of 16. Five studies of 1,180 patients examined relapse, defined as a return to the ED with worsening symptoms within 24 hours. The results found that the combined therapy group was less likely to have a return visit (RR = 0.80; 95% CI, 0.66 to 0.98) with an estimated 50 fewer relapses per 1,000 patients (200 vs. 250, respectively) for an NNT of 20.3
In the 11 studies (n = 1,392 patients) that considered adverse events, patients who received combined therapy were more likely to experience adverse events than those in the group receiving SABA monotherapy (odds ratio = 2.03; 95% CI, 1.28 to 3.20).3 This translates to 28 more adverse events per 1,000 patients treated (131 vs. 103, respectively) for a number needed to harm of 36. These adverse events were found to be self-limiting and relatively minor, including (but not limited to) tremors, agitation, and palpitations.
Caveats: The overall quality of the studies included in these trials was considered to be low to moderate. Many sources of bias existed in these studies, including that 14 studies did not disclose their source of funding. Additionally, only one-half of the studies disclosed methods clearly enough to be considered a true randomized controlled trial. Many of the studies included also had small sample sizes.
It is important to note that the benefit of a decrease in hospitalizations was found only in patients with severe asthma exacerbations, not those with mild to moderate exacerbations (test for difference between these subgroups found that P = .02). Regarding the decrease in relapse rates, there was heterogeneity in how this outcome was defined among the five studies analyzed (two studies defined relapse as return within 24 hours, one study as return within 48 hours, and two studies as return within two weeks). Furthermore, the authors were not able to examine potential differences in relapse rates among patients with mild, moderate, and severe exacerbations.
Fifteen studies compared hospitalization rates; of those, only five reported the criteria for admission. Interventions such as corticosteroid usage were clearly discussed in 13 studies; only in one-half did the study protocol dictate that corticosteroids be given to all participants, with the remainder of the trials leaving administration up to the physician. This may act as a confounder in these results.
Several studies attempted to evaluate improvement in forced expiratory volume in one second or percent increase above baseline, but the change in pulmonary function was not statistically significant between the two therapies and was deemed to be of low- or very-low-quality evidence in this review. However, 12 studies considered change in peak expiratory flow or percent improvement from baseline and found that patients given combined therapy did have an increase in both metrics compared with the SABA monotherapy group.
The description of adverse events across the studies was either too broad or not clearly defined, so it was not possible to extract specific adverse events for comparison.
Although the quality of evidence included in this review was low to moderate, the potential benefits of decreased hospitalization and relapse rates and the comparatively mild and limited adverse events suggest that patients presenting with acute asthma exacerbations are likely to benefit from combined short-acting anticholinergic and SABA therapy.
