
Am Fam Physician. 2018;97(11):714-715
Author disclosure: No relevant financial affiliations.
Clinical Question
Are cerebrospinal fluid (CSF) tau and ß-amyloid biomarkers accurate and practical tests for predicting which patients with mild cognitive impairment (MCI) will develop Alzheimer disease or other forms of dementia?
Evidence-Based Answer
There is insufficient evidence to support the routine use of CSF biomarkers for the detection of progressive dementias in patients with MCI. These tests carry the risk of overdiagnosis of dementia and, therefore, overtreatment. They have better sensitivity than specificity and may be more helpful at ruling out than ruling in progression to Alzheimer disease in patients with MCI.1 (Strength of Recommendation: B, based on inconsistent or limited-quality patient-oriented evidence.)
Practice Pointers
The initial symptoms in patients ultimately diagnosed with Alzheimer disease are often problems with planning, judgment, and memory, but they typically have preserved functionality in daily life. If formal testing confirms objective evidence of cognitive impairment, they are considered to have MCI. There are four potential outcomes for those with MCI: progression to Alzheimer disease, progression to another dementia, stable MCI, and recovery. Studies indicate that 6% to 15% of persons with MCI progress to Alzheimer disease each year.2 Currently, there is no clinical method to determine which patients with MCI will progress to Alzheimer disease or other forms of dementia. Growing evidence shows that measurable changes occur in the CSF of patients with MCI who are at risk of progression to Alzheimer disease or other variant dementias, which may allow for earlier intervention to delay the onset of dementia.3
The authors of this Cochrane review evaluated the diagnostic accuracy of CSF t-tau, CSF p-tau, the CSF t-tau:ß-amyloid ratio, and the CSF p-tau:ß-amyloid ratio index tests for detecting which patients with MCI at baseline would develop Alzheimer disease or other forms of dementia.1 The diagnosis of Alzheimer disease was made using various accepted definitions, including criteria from the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders.4 The review included 15 longitudinal cohort studies and 1,282 participants with MCI; 430 participants progressed to Alzheimer disease, and 130 participants progressed to other forms of dementia. Participants were 45 to 76 years of age, and follow-up ranged from one to four years. Because of the variation in index test thresholds, estimates of sensitivity and likelihood ratios were made at the fixed median value of specificity among the included studies.
The CSF p-tau:ß-amyloid ratio was the best test at ruling out conversion to Alzheimer disease, and the CSF t-tau:ß-amyloid ratio was the best test at ruling in conversion to Alzheimer disease, although there were only two studies. For example, consider 100 patients with MCI who undergo lumbar puncture and have these biomarkers assayed. Using a prevalence of 37%, a positive CSF t-tau result would correctly predict that 28 patients would develop Alzheimer disease, whereas nine patients who would develop Alzheimer disease would be missed (i.e., false negative). Eighteen would be misdiagnosed (i.e., false positive). In the same group, a positive CSF p-tau result would correctly predict that 30 patients would develop Alzheimer disease; there would be seven false-negative results and 33 false-positive results. A positive CSF p-tau:ß-amyloid ratio would correctly predict that 30 patients would develop Alzheimer disease, whereas there would be seven false-negative results and 22 false-positive results. Lastly, a positive CSF t-tau:ß-amyloid ratio would correctly predict that 34 to 36 patients would develop Alzheimer disease, and there would be one to three false-negative results and 31 or 32 false-positive results1 (Table 1).
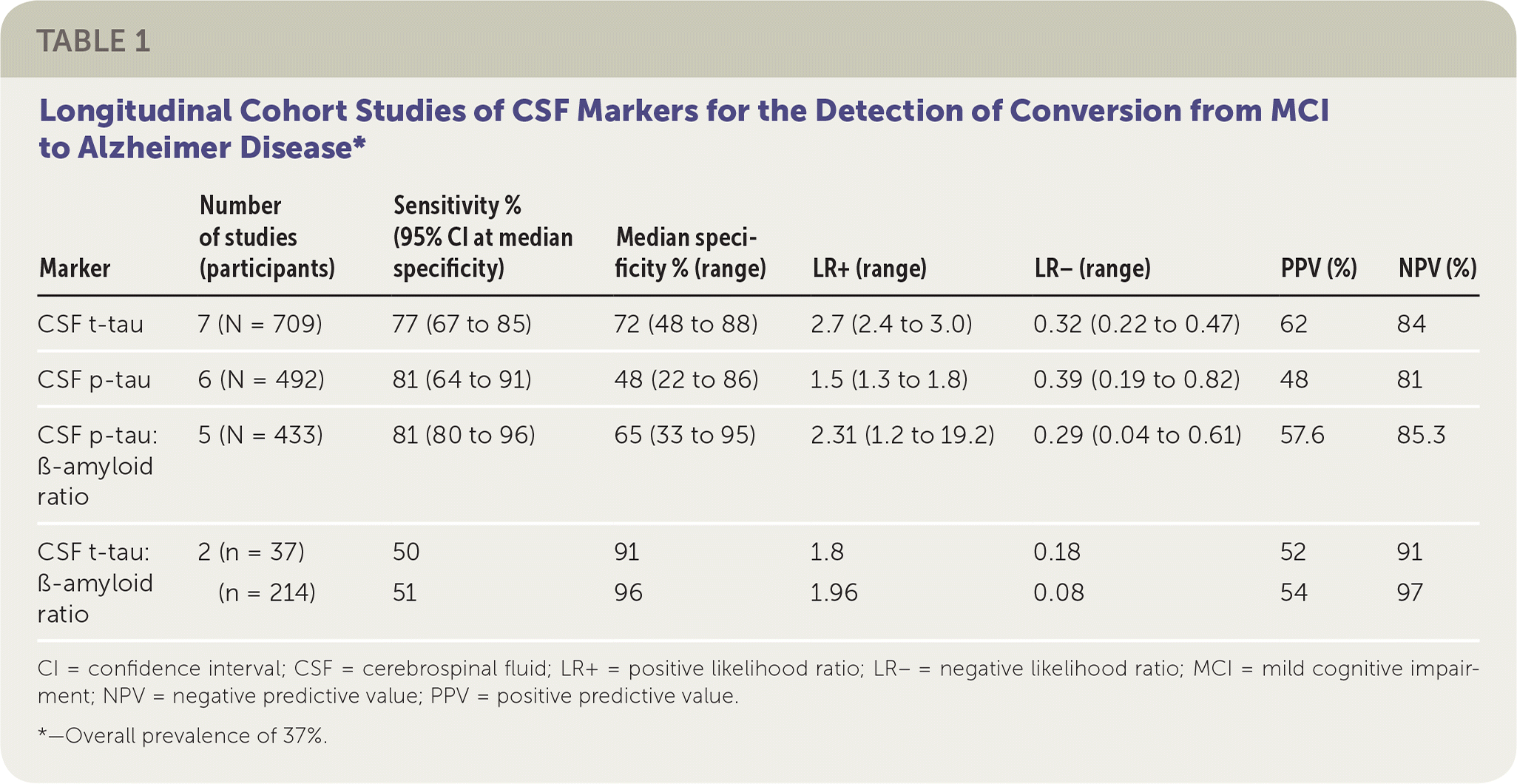
| Marker | Number of studies (participants) | Sensitivity % (95% CI at median specificity) | Median specificity % (range) | LR+ (range) | LR– (range) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|
| CSF t-tau | 7 (N = 709) | 77 (67 to 85) | 72 (48 to 88) | 2.7 (2.4 to 3.0) | 0.32 (0.22 to 0.47) | 62 | 84 | |
| CSF p-tau | 6 (N = 492) | 81 (64 to 91) | 48 (22 to 86) | 1.5 (1.3 to 1.8) | 0.39 (0.19 to 0.82) | 48 | 81 | |
| CSF p-tau: ß-amyloid ratio | 5 (N = 433) | 81 (80 to 96) | 65 (33 to 95) | 2.31 (1.2 to 19.2) | 0.29 (0.04 to 0.61) | 57.6 | 85.3 | |
| CSF t-tau: ß-amyloid ratio | 2 (n = 37) | 50 | 91 | 1.8 | 0.18 | 52 | 91 | |
| (n = 214) | 51 | 96 | 1.96 | 0.08 | 54 | 97 | ||
A meta-analysis was not conducted on the studies evaluating CSF p-tau:ß-amyloid ratio or CSF t-tau:ß-amyloid ratio because of the limited number of participants and heterogeneity. Overall, study quality was limited by poor reporting about how the clinical diagnosis of dementia was established, selection bias, inadequate blinding, variability in length of follow-up, and lack of a widely accepted threshold of the CSF diagnostic tests in patients with MCI.
The Biomarkers for Alzheimer's disease and Parkinson's disease European working group recommends the use of CSF Alzheimer disease biomarkers for the prediction of clinical progression or conversion to Alzheimer disease in patients with MCI with appropriate pre- and postbiomarker counseling.5 In the primary care setting, the utility of these invasive and expensive tests remains unclear because of the risk of overdiagnosis and lack of disease-modifying interventions that make early diagnosis beneficial.
The practice recommendations in this activity are available at http://www.cochrane.org/CD010803.
