
Am Fam Physician. 2018;97(12):online
Related Putting Prevention into Practice: Screening for Ovarian Cancer.
As published by the U.S. Preventive Services Task Force.
Summary of Recommendation and Evidence
The USPSTF recommends against screening for ovarian cancer in asymptomatic women (Table 1). D recommendation.
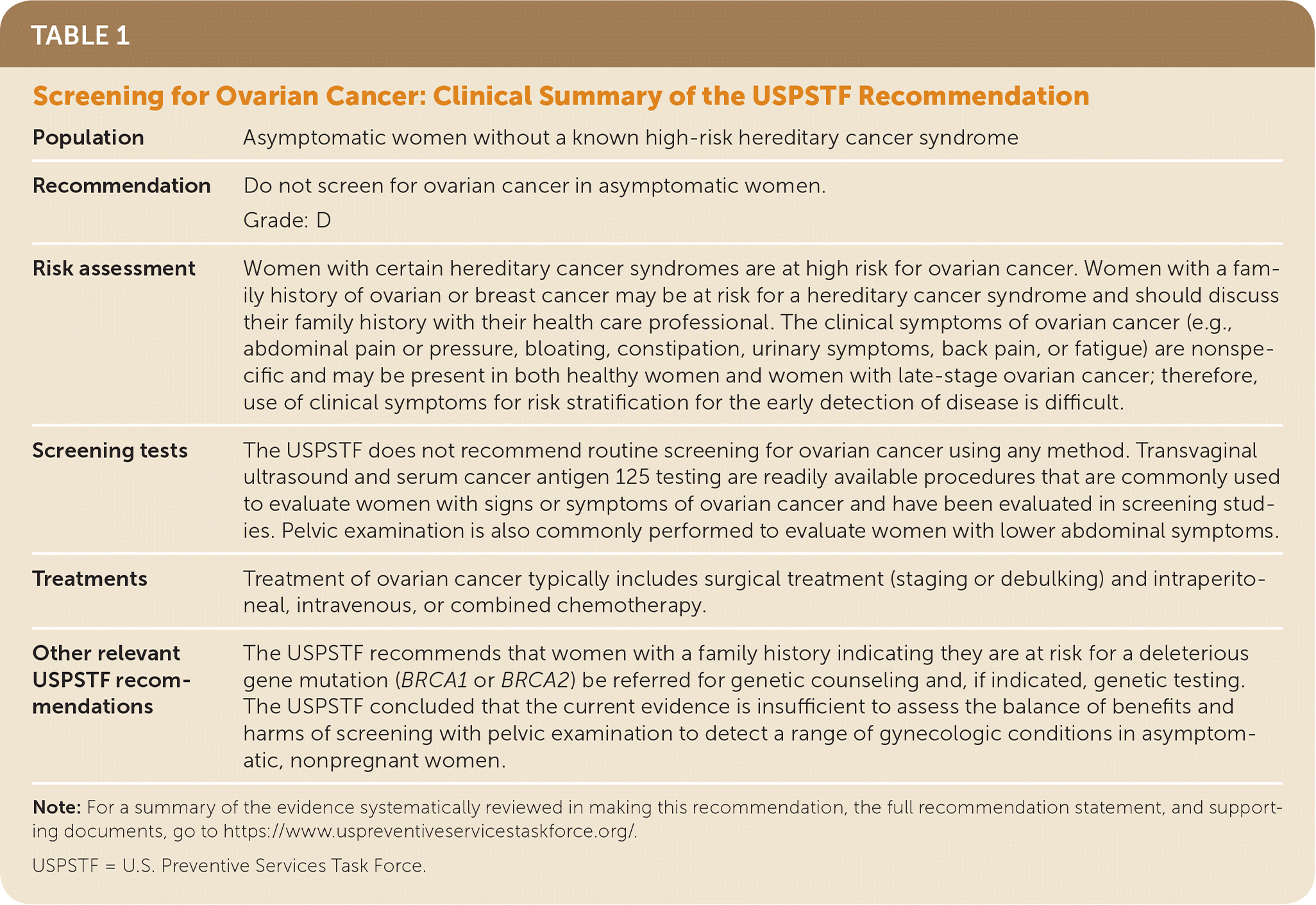
| Population | Asymptomatic women without a known high-risk hereditary cancer syndrome |
| Recommendation | Do not screen for ovarian cancer in asymptomatic women. |
| Grade: D | |
| Risk assessment | Women with certain hereditary cancer syndromes are at high risk for ovarian cancer. Women with a family history of ovarian or breast cancer may be at risk for a hereditary cancer syndrome and should discuss their family history with their health care professional. The clinical symptoms of ovarian cancer (e.g., abdominal pain or pressure, bloating, constipation, urinary symptoms, back pain, or fatigue) are nonspecific and may be present in both healthy women and women with late-stage ovarian cancer; therefore, use of clinical symptoms for risk stratification for the early detection of disease is difficult. |
| Screening tests | The USPSTF does not recommend routine screening for ovarian cancer using any method. Transvaginal ultrasound and serum cancer antigen 125 testing are readily available procedures that are commonly used to evaluate women with signs or symptoms of ovarian cancer and have been evaluated in screening studies. Pelvic examination is also commonly performed to evaluate women with lower abdominal symptoms. |
| Treatments | Treatment of ovarian cancer typically includes surgical treatment (staging or debulking) and intraperitoneal, intravenous, or combined chemotherapy. |
| Other relevant USPSTF recommendations | The USPSTF recommends that women with a family history indicating they are at risk for a deleterious gene mutation (BRCA1 or BRCA2) be referred for genetic counseling and, if indicated, genetic testing. The USPSTF concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening with pelvic examination to detect a range of gynecologic conditions in asymptomatic, nonpregnant women. |
This recommendation applies to asymptomatic women who are not known to have a high-risk hereditary cancer syndrome.
Rationale
IMPORTANCE
The age-adjusted incidence of ovarian cancer from 2010 to 2014 was 11.4 cases per 100,000 women per year.1 Ovarian cancer is the fifth most common cause of cancer death among U.S. women and the leading cause of death from gynecologic cancer, despite its low incidence.1 Approximately 14,000 women die of ovarian cancer each year in the United States. More than 95% of ovarian cancer deaths occur among women 45 years and older.2
DETECTION
The positive predictive value of screening tests for ovarian cancer is low, and most women with a positive screening test result do not have ovarian cancer (i.e., many women without ovarian cancer will have a false-positive result on screening tests).
BENEFITS OF SCREENING
The USPSTF found adequate evidence that screening with transvaginal ultrasound, testing for the serum tumor marker cancer antigen 125 (CA-125), or a combination of both does not reduce ovarian cancer mortality.
HARMS OF SCREENING
The USPSTF found adequate evidence that screening for ovarian cancer can result in important harms, including many false-positive results, which can lead to unnecessary surgical interventions in women who do not have cancer. Depending on the type of screening test used, the magnitude of harm ranges from moderate to substantial and reflects the risk for unnecessary diagnostic surgery. The USPSTF found inadequate evidence on the psychological harms of screening for ovarian cancer.
USPSTF ASSESSMENT
The USPSTF concludes that there is at least moderate certainty that the harms of screening for ovarian cancer outweigh the benefits.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to asymptomatic women who are not known to have a high-risk hereditary cancer syndrome. A hereditary cancer syndrome occurs when a genetic mutation is passed from parent to child that increases risk for developing cancers or can cause earlier onset of cancers. Women who have a hereditary cancer syndrome that puts them at high risk for ovarian cancer are excluded from this recommendation.
RISK ASSESSMENT
Women with certain hereditary cancer syndromes are at high risk for ovarian cancer. For example, women with BRCA1 or BRCA2 genetic mutations associated with hereditary breast and ovarian cancer are at high risk for ovarian cancer. Numerous genetic mutations and hereditary cancer syndromes may be associated with ovarian cancer, each with a different constellation of associated cancers and family history pattern.3–5 Women with a family history of ovarian or breast cancer may be at risk for a hereditary cancer syndrome and should discuss their family history with their health care professional. Management of a diagnosed hereditary cancer syndrome and prevention of ovarian cancer in these women is beyond the scope of this recommendation statement.
The clinical symptoms of ovarian cancer (e.g., abdominal pain or pressure, bloating, constipation, urinary symptoms, back pain, or fatigue) are nonspecific and may be present in both healthy women and women with late-stage ovarian cancer; therefore, use of clinical symptoms for risk stratification for the early detection of disease is difficult.
SCREENING TESTS
The USPSTF does not recommend routine screening for ovarian cancer using any method. Transvaginal ultrasound and serum CA-125 testing are readily available procedures that are commonly used to evaluate women with signs or symptoms of ovarian cancer, and both have been evaluated in screening studies. Pelvic examination is also commonly performed to evaluate women with lower abdominal symptoms, and although many clinicians perceive that pelvic examination with bimanual palpation of the ovaries is useful for screening for ovarian cancer,6 there is a lack of evidence to support this.7 Furthermore, the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial included bimanual palpation of the ovaries in its initial screening protocol, but this screening component was discontinued 5 years into the study because no cases of ovarian cancer were detected solely with bimanual palpation of the ovaries.8,9
The evaluation of abnormal test results consists of repeat testing with the same or a different test and often surgical removal (by laparoscopy or laparotomy) of 1 or both of the ovaries and fallopian tubes to determine whether a woman has ovarian cancer. Diagnostic guidelines recommend surgical removal of the complete ovary or ovaries, rather than tissue biopsy, to determine whether ovarian cancer is present.
TREATMENT
Treatment of ovarian cancer typically includes surgical treatment (staging or debulking) and intraperitoneal, intravenous, or combined chemotherapy.
USEFUL RESOURCES
In a separate recommendation statement, the USPSTF recommends that women with a family history indicating they are at risk for a deleterious gene mutation (BRCA1 or BRCA2) be referred for genetic counseling and, if indicated, genetic testing.10 The National Cancer Institute provides additional information on ovarian cancer risk and hereditary cancer syndromes.11 The USPSTF also concluded in a separate recommendation statement that the current evidence was insufficient to assess the balance of benefits and harms of screening with pelvic examination to detect a range of gynecologic conditions in asymptomatic, nonpregnant women.7
