Am Fam Physician. 2018;98(1):23-24
Author disclosure: No relevant financial affiliations.

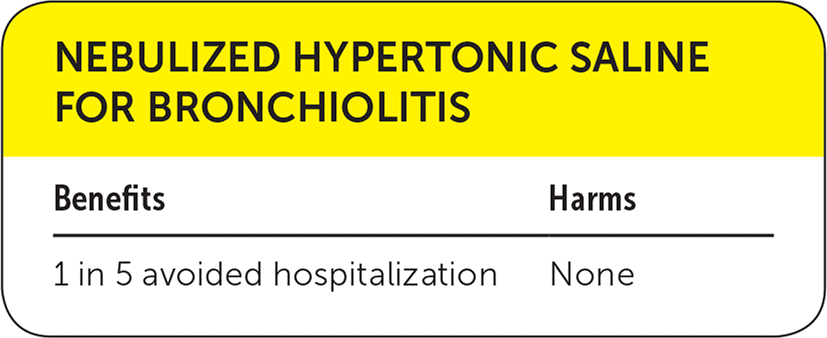
| Benefits | Harms |
|---|---|
| 1 in 5 avoided hospitalization | None |
Details for This Review
Study Population: Children younger than 24 months with bronchiolitis (3,209 participants in 24 trials). Most trials excluded patients who required mechanical ventilation, intensive care, or those who had oxygen saturation less than 85% on room air.
Efficacy End Points: Length of hospital stay, rate of hospitalization
Harm End Points: Tachycardia, hypertension, pallor, tremor, nausea, vomiting, diarrhea, and acute urinary retention
Narrative: Bronchiolitis is the most common lower respiratory tract infection in infants, with respiratory syncytial virus being the leading cause. Airway edema and mucus plugging are believed to be the pathologic processes causing morbidity in cases of viral bronchiolitis. Supportive treatment is the standard of care. In addition, nebulized hypertonic saline may be beneficial in relieving symptoms.
A systematic review included double-blind, randomized, controlled clinical trials evaluating the effect of nebulized hypertonic (3% or higher) saline solution alone or in conjunction with bronchodilators in infants with acute bronchiolitis compared with nebulized normal (0.9%) saline.1 Nebulized hypertonic saline resulted in a statistically significant reduction in length of hospital stay (mean difference: −0.45 day; 95% confidence interval [CI], −0.82 to −0.08). Nebulized hypertonic saline also reduced the risk of hospitalization by 20% compared with 0.9% saline (relative risk [RR] = 0.80; 95% CI, 0.67 to 0.96). No significant adverse events related to hypertonic saline inhalation were reported.
The lead author of this systematic review published a Cochrane review on the same topic in 2013.2 That meta-analysis showed a mean reduction of 1.2 days (95% CI, 0.8 to 1.5 days) in the length of hospital stay and no significant difference in the rate of hospitalization.2 We chose to write our summary based on the 2015 meta-analysis because it is more recent and includes several recent trials and approximately 2,000 more patients than the 2013 Cochrane review.
A 2014 meta-analysis reported an approximately one-day decrease in the length of hospital stay (weighted mean difference = −0.96; 95% CI, −1.38 to −0.54) in patients who received nebulized hypertonic saline compared with normal saline.3 This meta-analysis also showed a significant decrease in hospital admission rate (RR = 0.59; 95% CI, 0.37 to 0.93) after receiving nebulized hypertonic saline.
A 2017 randomized controlled trial enrolling 777 patients with bronchiolitis failed to show any significant difference in rate of hospital admission or length of hospital stay between the groups (nebulized hypertonic saline and nebulized normal saline).4
No significant adverse events related to hypertonic saline inhalation were observed in the trials reported in systematic reviews. No patients withdrew because of adverse events or clinical deterioration.
Caveats: The results of three large meta-analyses of randomized double-blind clinical trials suggest a high quality of evidence and show some benefits in using nebulized hypertonic saline compared with normal saline in children with bronchiolitis.1–3 However, the most concerning issue arising from reviewing the data is that most of the trials published after 2013, including two large multicenter European trails, have reported negative results.4–8 Heterogeneity among the trials and existence of effect modifiers could be responsible for this inconsistency.
The authors of the 2015 review offer an explanation for this discrepancy among the trials.1 A subgroup analysis performed by these authors found that trials in which virologic testing was available showed a significantly greater effect size of nebulized hypertonic saline (measured by reduction of length of hospital stay and rate of hospitalization) than trials without such testing. Thus, the diagnostic accuracy of bronchiolitis may affect the treatment outcomes.
The only consistent finding among all trials is the absence of any serious adverse event associated with the use of hypertonic saline. Considering the prevalence of bronchiolitis and the financial and emotional cost of hospitalization, possible reduction in admission rate and hospital stay with minimal adverse events provides enough evidence to recommend this treatment for acute bronchiolitis.
Most trials excluded patients who required mechanical ventilation or intensive care, and those who had oxygen saturation less than 85% on room air. Therefore, the findings of this review might not apply to infants with more severe bronchiolitis.
Despite reported benefits associated with the use of hypertonic saline for bronchiolitis, we have chosen a yellow recommendation because the more recent trials and systematic reviews have shown either much smaller effect size or no benefit at all compared with the older trials.
Copyright 2018 The NNT Group (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Assistant Medical Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/nebulized-hypertonic-saline-bronchiolitis/.