
Am Fam Physician. 2018;98(2):119-120
Author disclosure: No relevant financial affiliations.
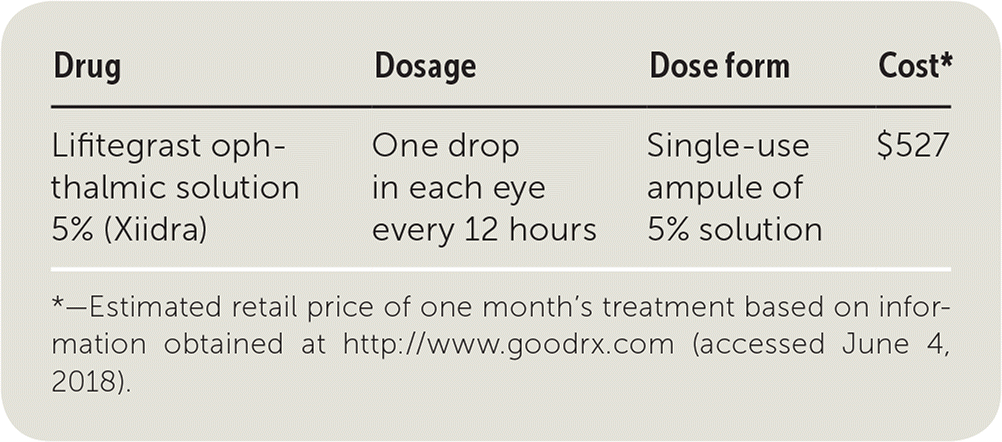
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Lifitegrast ophthalmic solution 5% (Xiidra) | One drop in each eye every 12 hours | Single-use ampule of 5% solution | $527 |
Safety
No serious adverse effects have been reported with the use of lifitegrast in clinical trials of up to one year in duration.2 Lifitegrast is not systemically detectable despite long-term administration, making systemic effects unlikely.1,2 The medication has not been studied in women who are pregnant or breastfeeding, children, or adolescents.1
Tolerability
Effectiveness
Lifitegrast has been evaluated in four 12-week randomized controlled trials involving a total of 2,132 patients.3 Patients receiving lifitegrast initially had moderate symptoms, an average score of 40 to 70 on a scale of 0 to 100, and some corneal lesions on fluorescein stain.3 Patients who received lifitegrast experienced a small reduction in symptoms (an average of 14 to 38 points), although these scores were only five to 12 points better than with placebo.3 Corneal lesions improved at a rate similar to that of placebo. Lifitegrast has not been evaluated in comparison with other treatments for dry eye disease or when used in combination with artificial tears.
Price
Lifitegrast costs around $527 for a one-month supply (one carton of 60 single-use ampules). This is similar to cyclosporine ophthalmic (Restasis), which costs approximately $529 for a one-month supply (one 5.5-mL bottle). Lifitegrast is much more expensive than over-the-counter treatments such as ointments, artificial tears, or lubricant eye drops. A 30-day supply of Refresh Plus lubricant eye drops costs about $24 (60 single-use ampules).
Simplicity
One drop of lifitegrast should be used in each eye twice a day (approximately 12 hours apart). It must be used regularly rather than as needed. Patients who wear contact lenses should remove them before applying lifitegrast drops. Lenses should not be reinserted until at least 15 minutes after administration of the drops.1 Each single-use ampule should be discarded immediately after use, and unused ampules should be stored in the original foil packaging to prevent exposure to light. Patients should wash hands before and after applying lifitegrast.
Bottom Line
Studies have shown that lifitegrast will reduce the symptoms of dry eye disease in some patients, although the benefit is borderline clinically significant. It is much more expensive than over-the-counter ointments, artificial tears, and lubricant eye drops. Lifitegrast may cause adverse effects in some patients. It should be considered only for patients with dry eye disease who have not found relief with other, less expensive treatments.
