Am Fam Physician. 2018;98(3):154-162
Patient information: See related handout on type 1 diabetes, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
There is considerable benefit of tight glucose control in patients with type 1 diabetes mellitus. Tight blood glucose control dramatically decreases the incidence of microvascular and macrovascular complications. Although glycemic goals should be individualized, most nonpregnant adults should strive for an A1C level less than 7%. Greater frequency of glucose monitoring and continuous glucose monitoring are both associated with lower A1C levels. The choice to monitor glucose levels via multiple daily capillary blood samples or continuous glucose monitoring is based on cost and patient preference. Intensive insulin treatment is recommended with a combination of multiple mealtime bolus and basal injections or with continuous insulin infusion through an insulin pump. The option to administer insulin with multiple daily injections vs. a pump should be individualized. Adjunctive medical therapy is under investigation but is not currently recommended. All patients with type 1 diabetes should participate in diabetes self-management education and develop individualized premeal insulin bolus plans under the guidance of a dietitian, if possible. Blood pressure and lipid control are important to prevent cardiovascular disease events. Patients with type 1 diabetes should have sick-day plans and be able to identify warning signs of hypoglycemia and diabetic ketoacidosis. Advances in diabetes care, including the bionic pancreas and the closed-loop system of glucose monitoring with an automated insulin pump, may have a significant effect on type 1 diabetes care in the years ahead.
The benefit of tight glucose control in patients with type 1 diabetes mellitus is well established.1–4 Microvascular complications (e.g., neuropathy, nephropathy, retinopathy) and macrovascular complications (e.g., myocardial infarction, cerebrovascular accident, cardiovascular disease– related deaths) are dramatically decreased when glucose levels are maintained as close to the nondiabetic range as possible.4 The numbers needed to treat with intensive therapy (A1C of approximately 7% vs. 9%) for a 10-year period to prevent progression of retinopathy and clinical neuropathy are 3 and 1.5, respectively.2 Additionally, intensive glycemic control reduces the risk of cardiovascular disease by 42% and severe cardiovascular events (nonfatal myocardial infarction, stroke, or death from cardiovascular disease) by 57% over 11 years among patients with type 1 diabetes.3 Long-term follow-up of the Diabetes Control and Complications Trial shows that the benefit of early, aggressive insulin therapy and intensive glycemic control persists for several decades after initiation of treatment. Although the exact pathophysiologic explanation for prolonged improved outcomes remains unclear, there is a decrease in all-cause mortality.5
WHAT IS NEW ON THIS TOPIC: TYPE 1 DIABETES
Long-term follow-up of the Diabetes Control and Complications Trial shows that the benefit of early, aggressive insulin therapy and intensive glycemic control persists for several decades after treatment and is associated with a decrease in all-cause mortality.
A well-designed double-blind randomized controlled trial of adults with type 1 diabetes who were taking metformin did not show significant improvement in glycemic control. The potential cardiovascular disease benefit remains under investigation.
In September 2016, the U.S. Food and Drug Administration approved the first combination glucose monitoring and automated insulin delivery device, a hybrid closed-loop system.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In persons with type 1 diabetes mellitus, self-monitoring blood glucose levels more frequently is recommended because it leads to improved A1C levels. | C | 8 |
| Basal-bolus insulin regimens are recommended for most persons with type 1 diabetes. | C | 14 |
| The decision to administer insulin via multiple daily injections or insulin pump can be individualized in persons with type 1 diabetes; neither method appears to be universally more effective. | C | 16 |
| In persons with type 1 diabetes, adjunctive treatment with metformin for improved glycemic control is not advised. | C | 25 |
| Regular education regarding sick day management and hypoglycemia should be provided to all persons with type 1 diabetes. | C | 35, 37 |
Glycemic Goals
Tight glycemic control remains the standard of care for most patients with type 1 diabetes. The American Diabetes Association recommends an A1C goal of less than 7% for non-pregnant adults (Table 1).6 Despite the benefits of lower A1C levels, goals should be personalized to account for individual preference, history of severe hypoglycemia, older age, and frailty. Higher glycemic targets for older adults or those with functional impairments, multiple comorbidities, or limited life expectancy are advisable.7

Self-Monitoring
There is a strong association between more frequent self-monitoring of blood glucose and lower A1C levels.8 The conventional approach, practiced by approximately 83% of patients with type 1 diabetes, is to monitor glucose levels via capillary blood testing.9 Testing is advised before meals, before exercise, before bedtime, occasionally postprandially, and anytime hypoglycemia is perceived.6 Although the optimal number of daily tests should be individualized, using these indications corresponds to six to 10 tests per day.
The newer practice of continuous glucose monitoring, used by approximately 17% of persons with type 1 diabetes, can also achieve tight glycemic control.9 With this method, a sensor inserted into the subcutaneous tissue measures interstitial glucose levels in real time and transmits them to a receiving device and monitor (Figure 1). The effectiveness of continuous glucose monitoring devices depends on adherence and does not completely eliminate the need for capillary testing, which is still required for device calibration and to confirm abnormal levels.
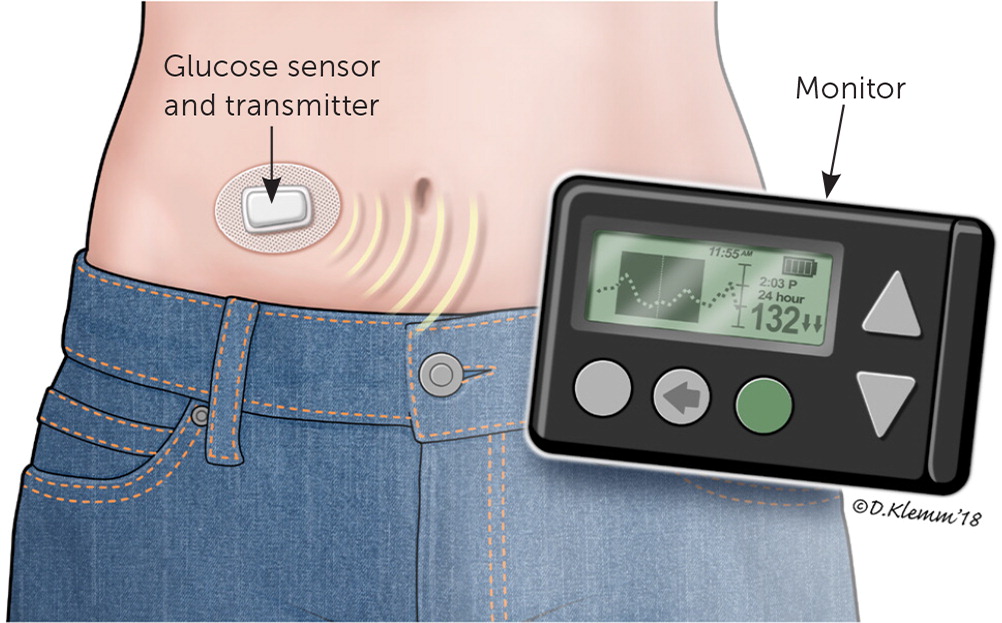
Compared with conventional self-monitoring, continuous glucose monitoring has been associated with improved glycemic control.10 One randomized controlled trial demonstrated a reduction in A1C levels from approximately 7.6% to 7.1% over six months in persons 25 years or older with type 1 diabetes who used continuous glucose monitoring, compared with traditional self-monitoring of blood glucose at least four times daily.11 Data do not show a definitive reduction in overall severe hypoglycemic events, but continuous glucose monitoring alarm features and trend alerts can notify patients and caregivers to expeditiously administer treatment.12 The significant increase in cost associated with continuous glucose monitoring needs to be considered when an individual is choosing between glucose monitoring approaches.13
Insulin Therapy
Consensus guidelines recommend intensive treatment with a combination of multiple mealtime bolus and basal injections or continuous insulin infusion through an insulin pump.14
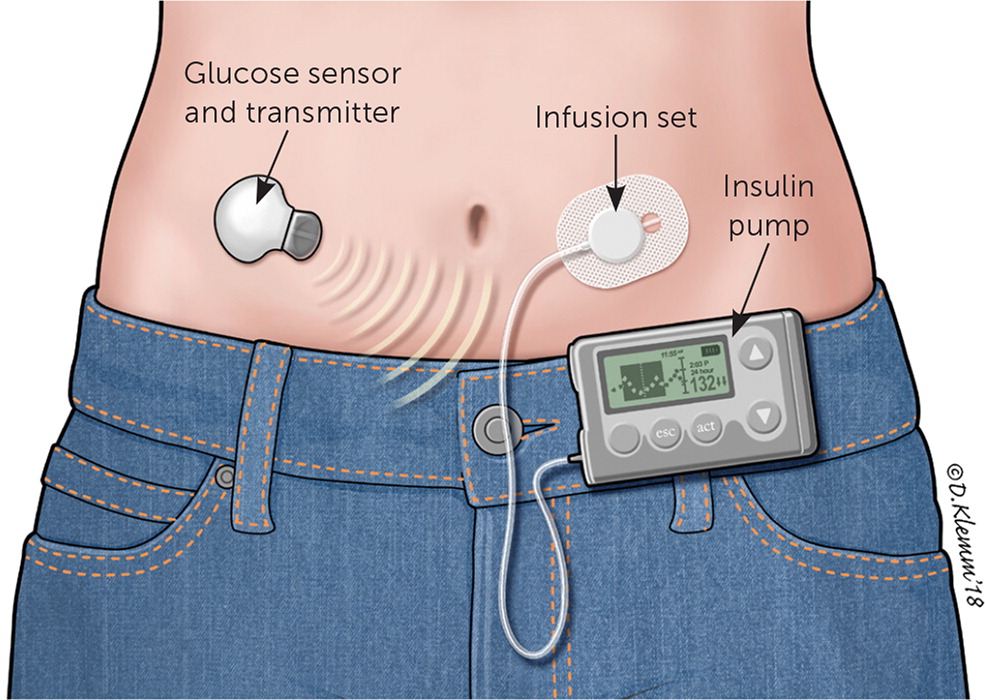
Approximately 64% of persons with type 1 diabetes in the United States use an insulin pump.9 The conventional portable insulin pump consists of an insulin reservoir, a computer chip to manage dosage delivery, and an infusion set with flexible tubing (Figure 2). The unit can be clipped onto a belt or waistband. A soft plastic cannula at the end of the tubing inserts under the skin to deliver the insulin. Alternatively, a patch pump attaches an entire unit directly to the skin, does not require a line of plastic tubing to deliver the insulin to the cannula, and receives insulin delivery programming via a wireless handheld unit. Regardless of the type of pump used, the cannula insertion site is rotated every two to three days. Despite the risks of lipodystrophy, cannula site discomfort, and increased cost, many patients still prefer the pump to performing multiple daily injections.
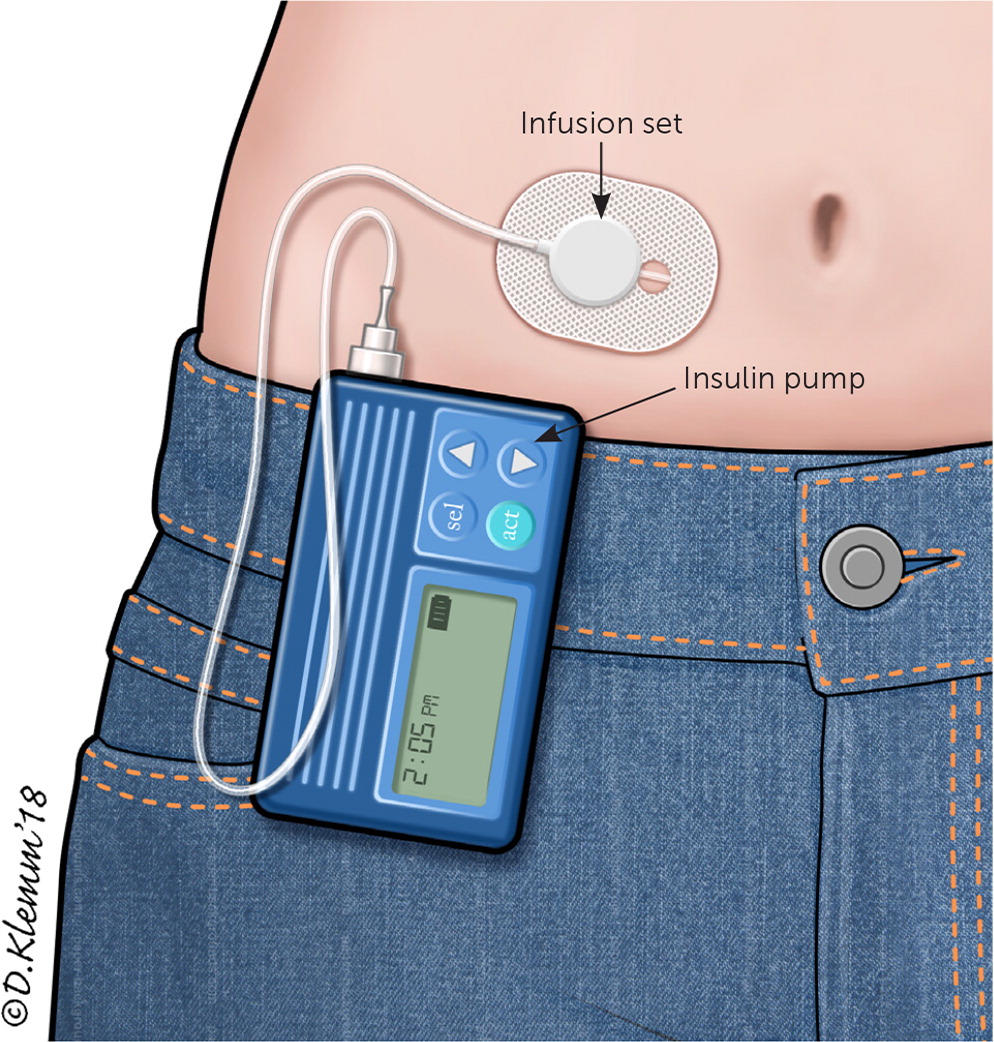
Basal-bolus regimens, whether through a pump or injections, are considered more physiologic because they attempt to mimic normal β-cell secretion. This is in contrast to the conventional insulin therapy used before the Diabetes Control and Complications Trial in which patients used mixed short- and intermediate-acting insulins in twice-daily dosing.15 Some studies have shown improved A1C levels in adults with type 1 diabetes who use continuous infusion pumps. However, studies have not shown better effectiveness universally for either approach. Therefore, the decision between multiple daily injections or continuous pump therapy should be individualized.16
Most patients who use the pump use rapid-acting insulin (e.g., aspart [Novolog], glulisine [Apidra], lispro [Humalog]), whereas a small minority still use regular insulin. Rapid-acting insulin boluses can be administered immediately before meals to allow more flexibility.17 Small amounts of rapid-acting insulin are continuously infused to provide basal insulin. Depending on pump type, basal rates can be titrated as low as 0.01 units per hour.18 Patients who do not use the pump tend to use long-acting insulin (e.g., detemir [Levemir], glargine [Lantus]) to meet basal demands.
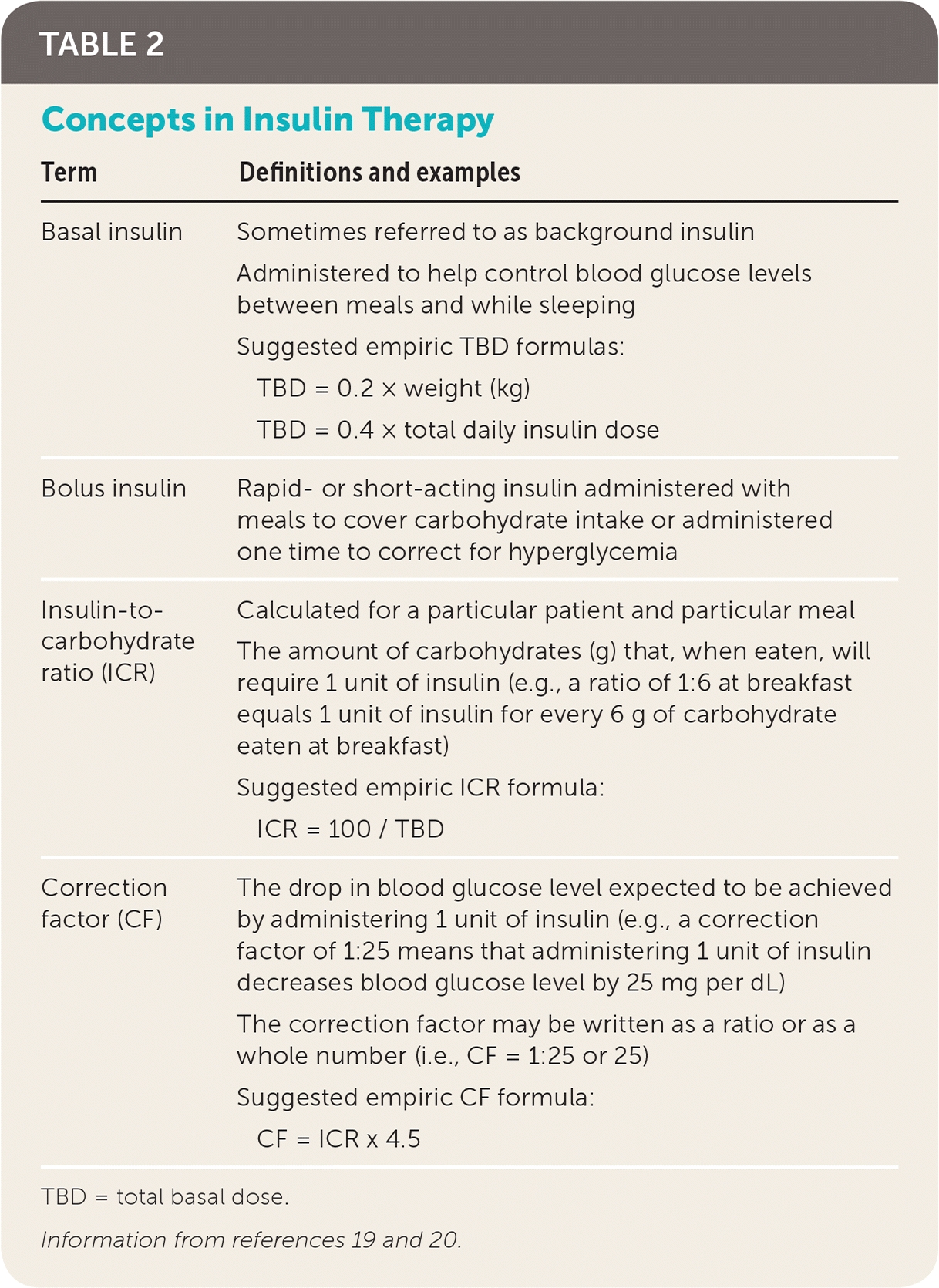
Intensive glucose control requires knowing the various factors affecting a patient's insulin sensitivity and dosing requirements (Table 2).19,20 Insulin sensitivity varies throughout the day and throughout a person's lifetime. Puberty, pregnancy, and illness are physiologic states that often require extra insulin titration. Before dosing of pre-meal insulin, consideration needs to be made regarding planned carbohydrate intake, planned exercise or activity levels, and current blood glucose levels (eTable A). Choice of insulin type needs to account for duration of action, cost, and route of administration15,21 (eTable B).
| Term | Definitions and examples |
|---|---|
|
|
| |
| |
|
|
|
|
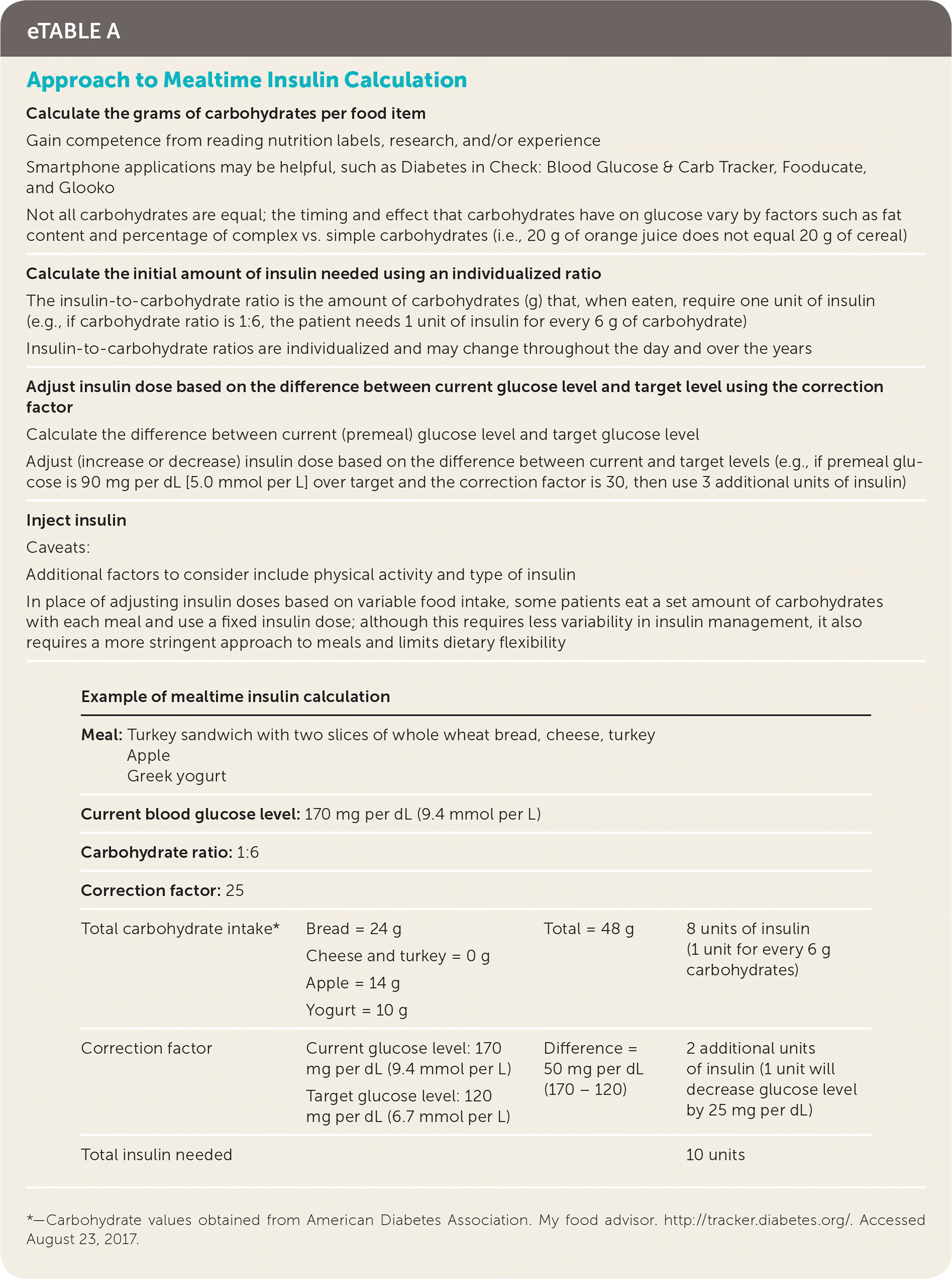
| Calculate the grams of carbohydrates per food item | |||
| Gain competence from reading nutrition labels, research, and/or experience Smartphone applications may be helpful, such as Diabetes in Check: Blood Glucose & Carb Tracker, Fooducate, and Glooko Not all carbohydrates are equal; the timing and effect that carbohydrates have on glucose vary by factors such as fat content and percentage of complex vs. simple carbohydrates (i.e., 20 g of orange juice does not equal 20 g of cereal) | |||
| Calculate the initial amount of insulin needed using an individualized ratio | |||
| The insulin-to-carbohydrate ratio is the amount of carbohydrates (g) that, when eaten, require one unit of insulin (e.g., if carbohydrate ratio is 1:6, the patient needs 1 unit of insulin for every 6 g of carbohydrate) Insulin-to-carbohydrate ratios are individualized and may change throughout the day and over the years | |||
| Adjust insulin dose based on the difference between current glucose level and target level using the correction factor | |||
| Calculate the difference between current (premeal) glucose level and target glucose level Adjust (increase or decrease) insulin dose based on the difference between current and target levels (e.g., if premeal glucose is 90 mg per dL [5.0 mmol per L] over target and the correction factor is 30, then use 3 additional units of insulin) | |||
| Inject insulin | |||
| Caveats: | |||
| Additional factors to consider include physical activity and type of insulin In place of adjusting insulin doses based on variable food intake, some patients eat a set amount of carbohydrates with each meal and use a fixed insulin dose; although this requires less variability in insulin management, it also requires a more stringent approach to meals and limits dietary flexibility | |||
| Example of mealtime insulin calculation | |||
Meal:
| |||
| Current blood glucose level: 170 mg per dL (9.4 mmol per L) | |||
| Carbohydrate ratio: 1:6 | |||
| Correction factor: 25 | |||
| Total carbohydrate intake* | Bread = 24 g Cheese and turkey = 0 g Apple = 14 g Yogurt = 10 g | Total = 48 g | 8 units of insulin (1 unit for every 6 g carbohydrates) |
| Correction factor | Current glucose level: 170 mg per dL (9.4 mmol per L) Target glucose level: 120 mg per dL (6.7 mmol per L) | Difference = 50 mg per dL (170 – 120) | 2 additional units of insulin (1 unit will decrease glucose level by 25 mg per dL) |
| Total insulin needed | 10 units | ||
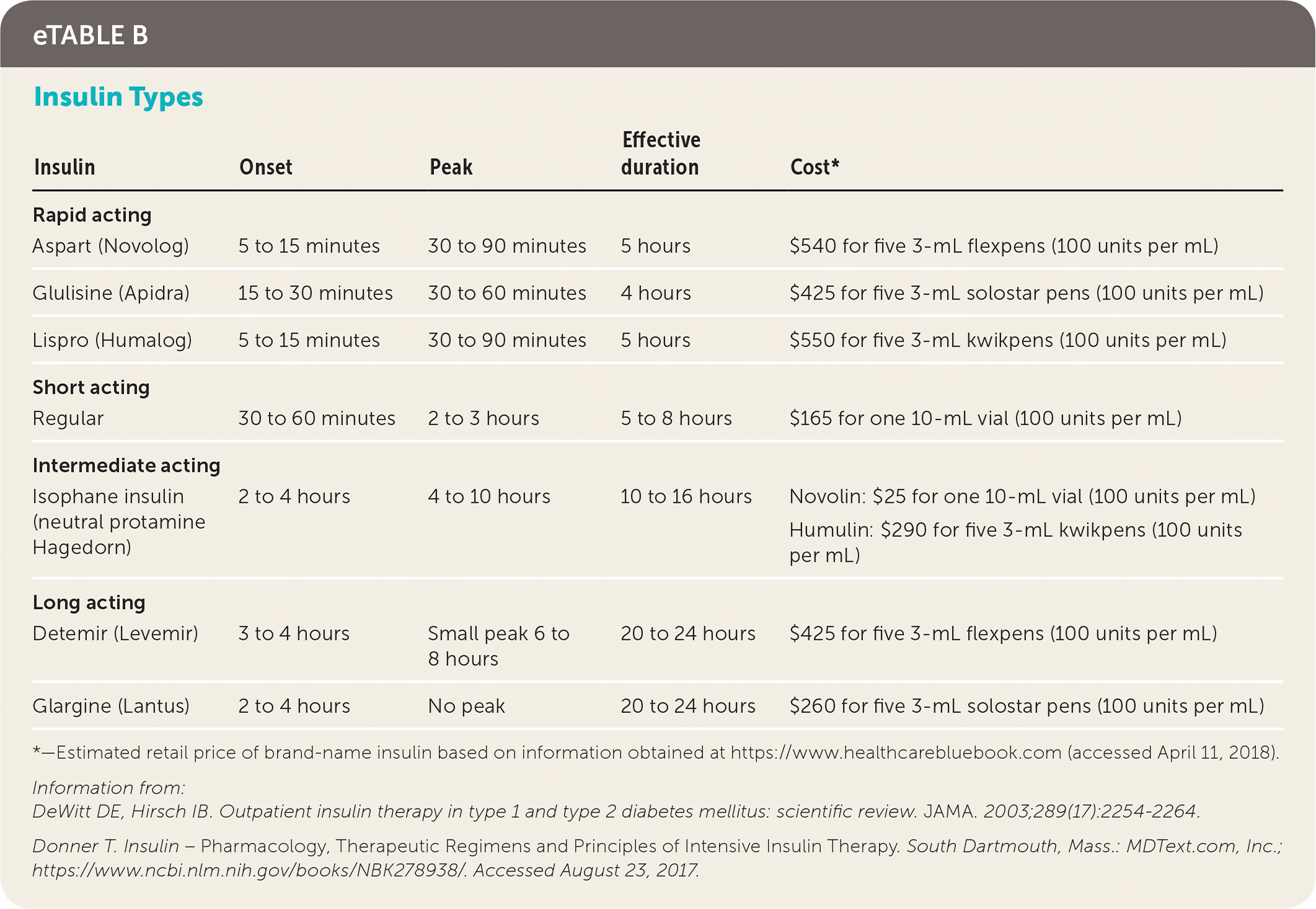
| Insulin | Onset | Peak | Effective duration | Cost* |
|---|---|---|---|---|
| Rapid acting | ||||
| Aspart (Novolog) | 5 to 15 minutes | 30 to 90 minutes | 5 hours | $540 for five 3-mL flexpens (100 units per mL) |
| Glulisine (Apidra) | 15 to 30 minutes | 30 to 60 minutes | 4 hours | $425 for five 3-mL solostar pens (100 units per mL) |
| Lispro (Humalog) | 5 to 15 minutes | 30 to 90 minutes | 5 hours | $550 for five 3-mL kwikpens (100 units per mL) |
| Short acting | ||||
| Regular | 30 to 60 minutes | 2 to 3 hours | 5 to 8 hours | $165 for one 10-mL vial (100 units per mL) |
| Intermediate acting | ||||
| Isophane insulin (neutral protamine Hagedorn) | 2 to 4 hours | 4 to 10 hours | 10 to 16 hours | Novolin: $25 for one 10-mL vial (100 units per mL) |
| Humulin: $290 for five 3-mL kwikpens (100 units per mL) | ||||
| Long acting | ||||
| Detemir (Levemir) | 3 to 4 hours | Small peak 6 to 8 hours | 20 to 24 hours | $425 for five 3-mL flexpens (100 units per mL) |
| Glargine (Lantus) | 2 to 4 hours | No peak | 20 to 24 hours | $260 for five 3-mL solostar pens (100 units per mL) |
Adjunctive Therapies
Adjunctive therapies for patients with type 1 diabetes are under investigation but are not currently recommended.22 Sodium glucose cotransporter-2 inhibitors, which promote the renal excretion of glucose, are approved for use in patients with type 2 diabetes but have not been approved for use in patients with type 1 diabetes. Pramlintide (Symlin)—a synthetic analogue of human amylin that reduces postprandial glucose via slowed gastric emptying, inhibition of glucagon secretion, and satiety promotion—is approved by the U.S. Food and Drug Administration for use in type 1 diabetes and may have an association with improved glycemic control; however, long-term benefits remain unclear.23 Despite studies suggesting that the addition of metformin might reduce insulin doses in patients with type 1 diabetes,24 this has not been substantiated. Additionally, a recent well-designed double-blind randomized controlled trial of adults with type 1 diabetes taking metformin did not show significant improvement in glycemic control.25 The potential cardiovascular disease benefit from adjunctive therapies remains under investigation.
Lifestyle Management and Disease Prevention
DIABETES SELF-MANAGEMENT EDUCATION
All patients with type 1 diabetes should participate in continuous diabetes self-management education, which works to empower patients to understand how diet, physical activity, and insulin affect their glucose levels and how glycemic levels relate to acute and chronic complications (Table 3).6,26–29 Diabetes self-management education has been shown to improve A1C levels and quality of life, and reduce health care costs.30,31 National standards for diabetes self-management education have been published by the American Diabetes Association in conjunction with the American Association of Diabetes Educators.32 Online diabetes education resources are available at http://professional.diabetes.org/content/diabetes-educator-resources. To find a diabetes educator or a preexisting diabetes education program, visit http://www.diabeteseducator.org/living-with-diabetes.
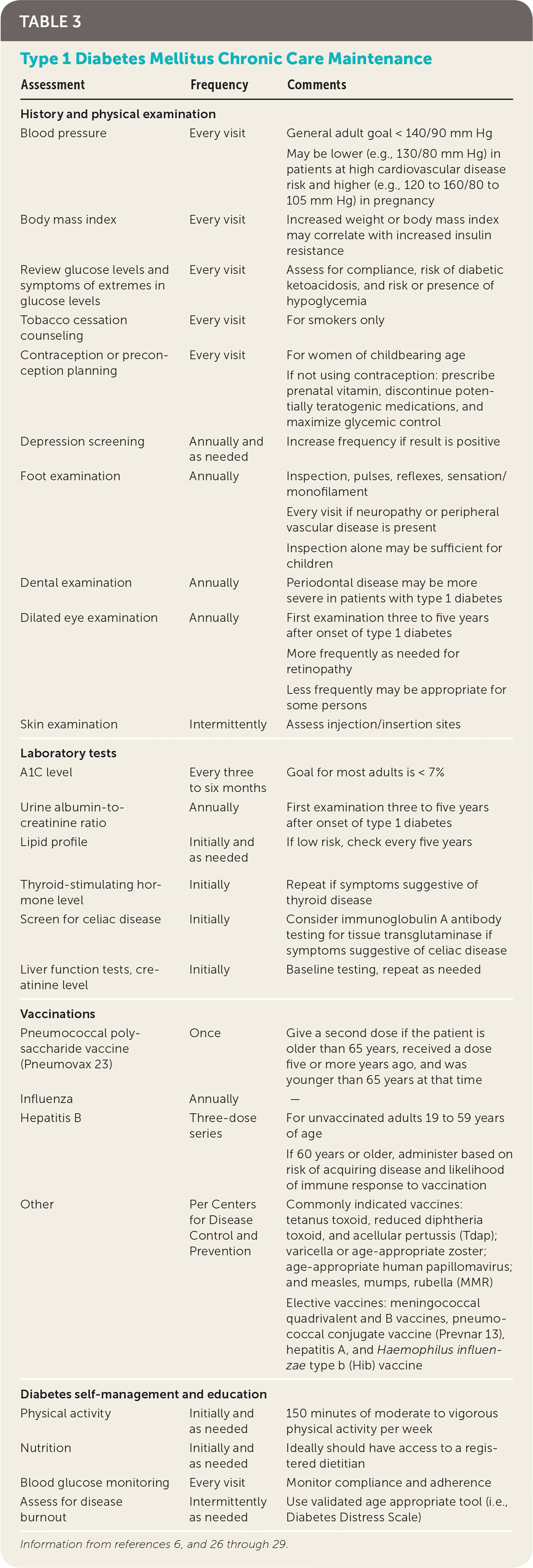
| Assessment | Frequency | Comments |
|---|---|---|
| History and physical examination | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Laboratory tests | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vaccinations | ||
|
|
|
|
|
|
|
|
|
|
|
|
| Diabetes self-management and education | ||
|
|
|
|
|
|
|
|
|
|
|
|
MOTIVATIONAL INTERVIEWING
Motivational interviewing techniques can be an effective strategy for improving glycemic control. One randomized controlled trial demonstrated that adolescents with newly diagnosed type 1 diabetes were able to decrease their A1C level by an average of 0.6% over 12 months (compared with an increase of 0.2% in the control group) by participating in motivational interviewing sessions every six to eight weeks.33 For resources on motivational interviewing specifically for diabetes, visit https://www.niddk.nih.gov/health-information/communication-programs/ndep/health-professionals/practice-transformation-physicians-health-care-teams/diabetes-practice-changes/engaging-patients/motivational-interviewing.
NUTRITION
Nutritional therapy should be individualized and supervised under the care of a dietitian. Matching carbohydrate intake with insulin therapy and activity level is a complex practice. One approach to managing mealtime insulin is to eat a set amount of carbohydrates with each meal and use a fixed insulin dose. A second approach is to match insulin doses according to variable amounts of carbohydrates that one plans to consume. Alcohol intake should be restricted to no more than one drink per day for women and two drinks per day for men.
PHYSICAL ACTIVITY
The American Diabetes Association suggests that adults with type 1 diabetes should engage in 150 minutes of moderate- to vigorous-intensity physical activity per week with no more than two consecutive days without activity.26 Prolonged sitting should be interrupted every 30 minutes with light activity.34
CARDIOVASCULAR DISEASE PREVENTION
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality among persons with diabetes.27 Particular attention should be given to maximizing blood pressure and lipid control (Table 4).27 Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers should be used preferentially in patients with diabetes who have hypertension and microalbuminuria (urinary albumin-to-creatinine ratio greater than 30 mg per g).27

| Blood pressure |
| Measure at each visit; review therapeutic lifestyle changes if > 120/80 mm Hg |
| Medical treatment to maintain goal < 140/90 mm Hg (may be lower [e.g., 130/80 mm Hg] in patients at high risk of cardiovascular disease and higher [e.g., 120 to 160/80 to 105 mm Hg] in pregnancy) |
| Treatment should focus on drugs proven to reduce cardiovascular events in patients with diabetes (angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, thiazides, and dihydropyridine calcium channel blocker) |
| Cholesterol |
| Measure at initial evaluation and at least every five years |
| Few clinical trials exist studying cholesterol and type 1 diabetes |
| Primary prevention: in the absence of ASCVD, consider statin therapy based on ASCVD risk scores, such as the American College of Cardiology/American Heart Association risk assessment tool (https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp) |
| Secondary prevention: treat with statins if ASCVD is present |
| Antiplatelet therapy |
| Primary prevention: in the absence of ASCVD, consider aspirin therapy (75 to 162 mg daily) for patients older than 50 years with one additional risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, or albuminuria) who are not at increased risk of bleeding |
| Secondary prevention: treat with aspirin (75 to 162 mg daily) if ASCVD is present |
| Smoking cessation |
| Ask about smoking at each visit |
| Advise all patients to not smoke cigarettes or use tobacco products |
| Offer counseling and tobacco cessation treatment as routine care |
Acute Issues of Type 1 Diabetes
HYPOGLYCEMIA
Clinically significant hypoglycemia is defined as a plasma glucose level less than 54 mg per dL (3.0 mmol per L) with or without symptoms. Severe hypoglycemia is defined as a hypoglycemic event in which a patient requires the assistance of another individual for treatment. A blood glucose level of less than 70 mg per dL (3.9 mmol per L) is considered a “glucose alert level” and should trigger treatment with fast-acting carbohydrates and consideration for changes in medication.6,35 The risk of severe hypoglycemia, as demonstrated by the Diabetes Control and Complications Trial, increases with intensive diabetes treatment and lower A1C levels.1 More recent data suggest that as glucose monitoring techniques and insulin types become more sophisticated, this pattern may be changing, and the rate of severe hypoglycemia may not be greater in those with tight glucose control.9
Hypoglycemia symptoms vary in severity, and range from hunger and confusion to loss of consciousness, seizure, and death. An individual's physiologic response to hypoglycemia depends on the frequency of hypoglycemic events.36 For example, patients who experience frequent episodes of hypoglycemia may have less severe symptoms at the same glucose levels as patients who rarely experience hypoglycemia. Patients must maintain a tight balance between providing sufficient iatrogenic (exogenous) insulin to prevent hyperglycemia but not so much as to cause hypoglycemia. Regardless of the amount of insulin administered, the risk of recurrent hypoglycemia is exacerbated by decreased glucose counter regulation (epinephrine and glucagon response) and hypoglycemic unawareness.36
Recommended treatment for hypoglycemia is 15 g of oral glucose. Any type of carbohydrate can increase blood glucose levels; however, complex carbohydrates and increased fat content can delay acute resolution of a hypoglycemic event. Repeat administration of glucose may be required. Glucagon (Glucagen) can be delivered by injection to an unconscious patient with hypoglycemia. Patients and their caregivers should understand the symptoms of hypoglycemia and how to administer proper treatment with glucagon.
HYPERGLYCEMIA
One of the most serious acute complications of type 1 diabetes is diabetic ketoacidosis (DKA). Precipitating factors of DKA include infection, discontinuation or inadequate administration of insulin (including insulin pump failure), myocardial infarction, and other drugs. Prevention strategies for DKA include ensuring adequate access to supplies and prescriptions, and education regarding sick-day management. A previous article from American Family Physician on DKA management is available at https://www.aafp.org/afp/2013/0301/p337.html.
SICK DAYS
Patients often require increased amounts of insulin during acute illness. Patients experiencing acute illness should increase the frequency of glucose testing and should not self-discontinue insulin (Table 5).14,37 Frequent administration of rapid-acting insulin every two to four hours based on glucose levels may be required. In the setting of vomiting or hyperglycemia, ketone testing can help guide management.37 Recognizing early signs and symptoms of dehydration and DKA is important. Patients and caregivers should contact their physician as soon as DKA is suspected.
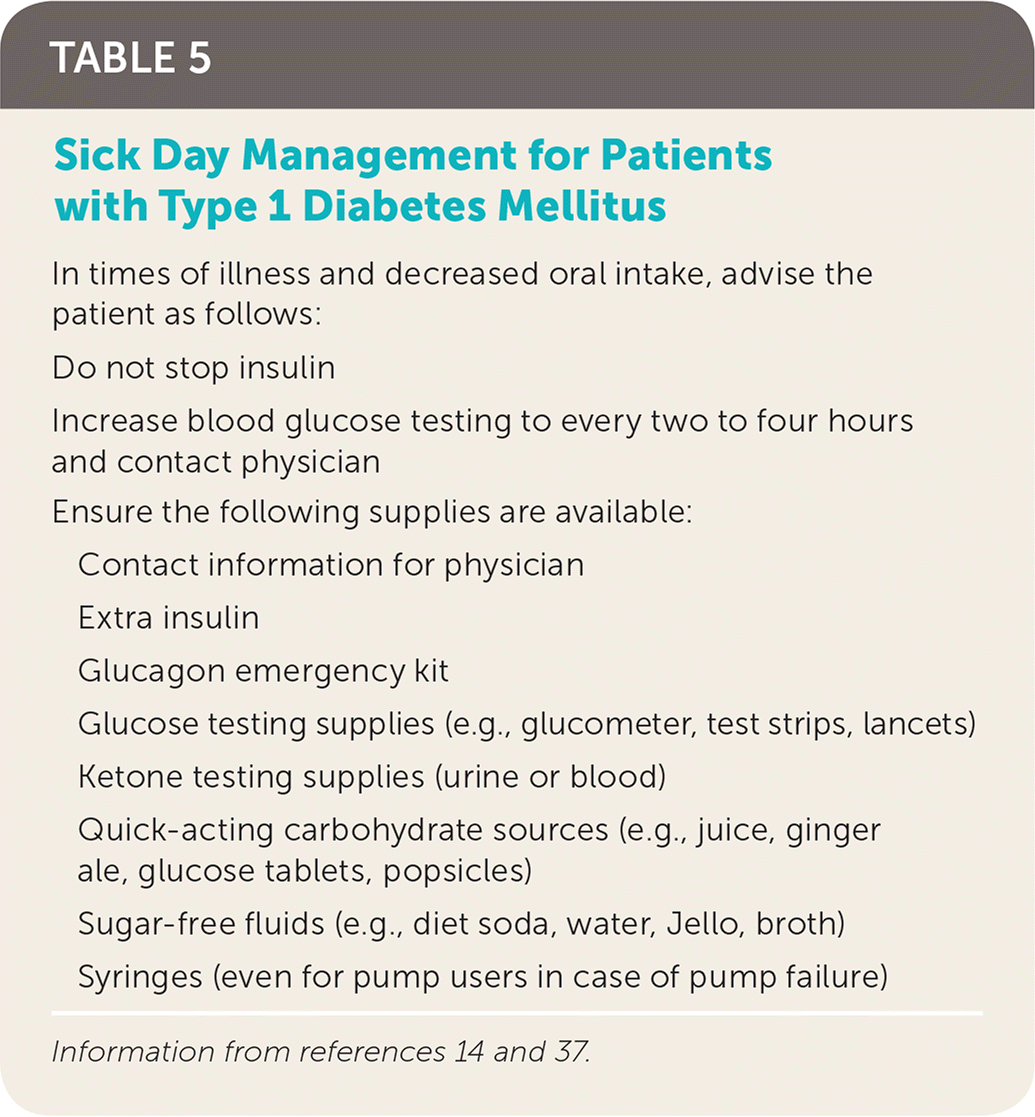
| In times of illness and decreased oral intake, advise the patient as follows: | |
| Do not stop insulin | |
| Increase blood glucose testing to every two to four hours and contact physician | |
| Ensure the following supplies are available: | |
| Contact information for physician | |
| Extra insulin | |
| Glucagon emergency kit | |
| Glucose testing supplies (e.g., glucometer, test strips, lancets) | |
| Ketone testing supplies (urine or blood) | |
| Quick-acting carbohydrate sources (e.g., juice, ginger ale, glucose tablets, popsicles) | |
| Sugar-free fluids (e.g., diet soda, water, Jello, broth) | |
| Syringes (even for pump users in case of pump failure) | |
Future Directions
Technologic advances have helped improve the usability of continuous glucose monitors and insulin pumps. Various models and manufacturers allow for choice based on size, insertion type, and tubing.
Current research is focused on improving communication between devices and more automation of insulin delivery based on recorded blood glucose levels. In September 2016, the U.S. Food and Drug Administration approved the first combination glucose monitoring and automated insulin delivery device, a hybrid closed-loop system.38 Some commercially available closed-loop systems use a continuous glucose monitor, which transmits directly to the insulin pump (Figure 3).
In addition to the closed-loop system, an automated, bihormonal (insulin and glucagon) bionic pancreas is under development. Studies of the bihormonal system have shown improved glycemic control and reduced hypoglycemia during testing.39
Pancreas and islet cell transplantation has also been effective in restoring insulin production and normalizing glucose levels. However, it requires lifelong immunosuppressive therapy. Therefore, it is currently recommended only for patients who also require renal transplantation.40
Several recent advances in type 1 diabetes research have been driven by improved patient databases. The T1D Exchange, which includes more than 30,000 registry participants, manages the largest registry of patients with type 1 diabetes in the United States. Table 6 provides a list of key findings from the T1D Exchange registry.9

| 1. Children and adults with excellent glycemic control manage their diabetes differently than persons who have poorer glycemic control. Differences in management include using specific protocols and systems to decide amount of insulin dosed and when to give boluses, and increased frequency of monitoring and exercise. |
| 2. Self-monitoring blood glucose levels more frequently is strongly associated with better glycemic control. |
| 3. There are racial and socioeconomic disparities among those who use insulin pumps and those who have better glucose control. Within the T1D Exchange, pump use is more common in whites than blacks or Hispanics, even when adjusting for socioeconomic status; blacks have higher A1C levels than whites or Hispanics. |
| 4. A1C levels are lower in persons who practice continuous glucose monitoring vs. those who do not. This finding is shown when continuous glucose monitoring is used regularly in patients using either multiple daily injections or an insulin pump. |
| 5. Severe hypoglycemia is more common in older adults (> 60 years) with longstanding type 1 diabetes. |
| 6. The rate of severe hypoglycemia is not higher in persons with tight glucose control (A1C < 7%) vs. persons who have poorer glucose control (A1C > 8%) This is in contrast to the findings in the DCCT, which reported higher rates of severe hypoglycemia in patients with tight glucose control. A proposed explanation for this dramatic change is the improvements in insulin and glucose monitoring since the DCCT in 1983–1993. |
| 7. DKA is not more common in persons who use insulin pumps than those who use injections. Historically, there were concerns that DKA occurred more often in pump users because of undetected failure of the pump. |
| 8. Adolescents and young adults with type 1 diabetes have worse glucose control and experience DKA more often than younger or older individuals with type 1 diabetes. This represents an area for increased vigilance and research around behavior modification and improved diabetes self-management education. |
| 9. Adolescents with type 1 diabetes are more overweight than adolescents without type 1 diabetes. Obesity increases the risk of insulin resistance, severe hypoglycemia, and cardiovascular disease. |
| 10. Microalbuminuria is an early sign of kidney disease and an important warning sign. Registry data show the relationship of microalbuminuria with glucose control, diabetes duration, and blood pressure, and highlight the need to focus on glycemic control to prevent chronic kidney disease. |
This article updates previous articles on this topic by Havas and Donner41 and Havas.42
Data Sources: A literature search was completed in Medline via Ovid, EBSCOhost, DynaMed, and the Cochrane Database of Systematic Reviews using the following keywords: type 1 diabetes, management of diabetes, insulin therapy, and glucose monitoring. Additionally, the Essential Evidence Plus evidence summary literature search sent by the AFP medical editors was reviewed. Search dates: July 3 and August 21, 2017, and May 2018.