Am Fam Physician. 2018;98(3):164-170
Related letter: Additional Rheumatologic Tests Critical in Patients with Systemic Sclerosis
Author disclosure: No relevant financial affiliations.
Patients with a suspected connective tissue disorder should undergo serologic testing to confirm the diagnosis and, in some cases, to monitor disease activity and predict flares. Patients with suspected systemic lupus erythematosus should be tested for antinuclear antibodies. However, antinuclear antibodies are not specific and may be present in many other connective tissue disorders and nonrheumatologic diseases. Thus, patients with suspected systemic lupus erythematosus should undergo further testing to confirm the diagnosis. Patients with Sjögren syndrome may have a positive antinuclear antibody titer, but often also have positive anti-Sjögren antigen A or B results. Similarly, antinuclear antibodies can be present in patients with scleroderma, mixed connective tissue disease, and dermatomyositis or polymyositis. Additional tests are needed to help confirm the diagnosis. In patients with findings of rheumatoid arthritis, a positive rheumatoid factor titer suggests the diagnosis, but as with antinuclear antibodies, it is not specific and can occur in other conditions. Rheumatoid factor can also be negative in patients with rheumatoid arthritis. A positive anticyclic citrullinated peptide antibody titer is more specific for rheumatoid arthritis and can help confirm the diagnosis. Physicians should order these serologic tests only when patients have a high pretest probability of a specific connective tissue disorder.
This article gives a framework for laboratory testing in patients with a suspected connective tissue disease. Common diseases are presented with typical symptoms, and an overview of appropriate testing is provided.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In patients with a suspected connective tissue disorder and a positive ANA titer, further testing (e.g., anti–double-stranded DNA antibodies, anti-Smith antibodies, Sjögren antibodies) should be performed based on clinical findings that raise suspicion for specific disorders. | C | 9 |
| Testing for antineutrophil cytoplasmic antibodies is not indicated in the evaluation of patients with sinusitis who have no other features of vasculitis. | C | 29, 30 |
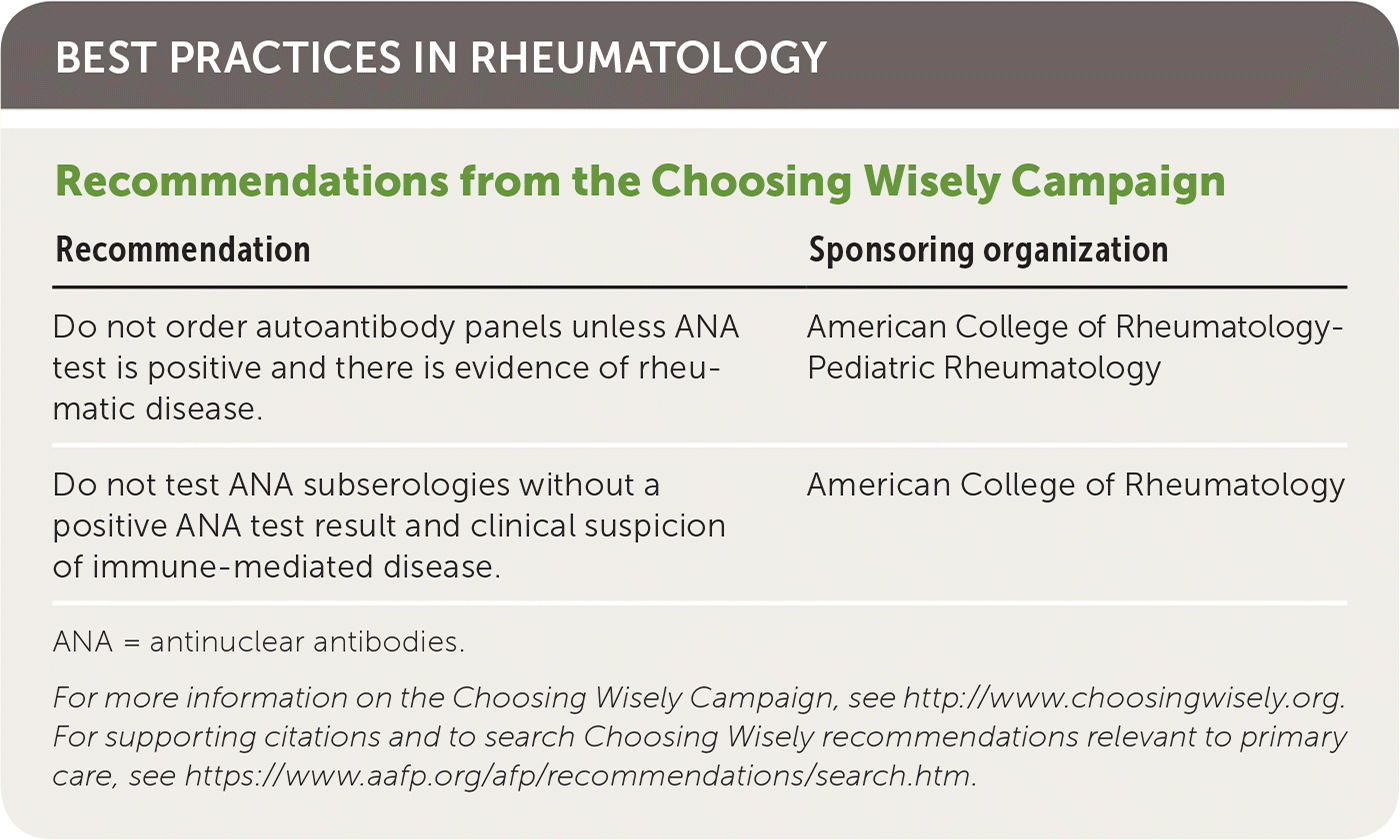
| Recommendation | Sponsoring organization |
|---|---|
| Do not order autoantibody panels unless ANA test is positive and there is evidence of rheumatic disease. | American College of Rheumatology-Pediatric Rheumatology |
| Do not test ANA subserologies without a positive ANA test result and clinical suspicion of immune-mediated disease. | American College of Rheumatology |
Clinical Scenario
A 28-year-old woman presents with a three-month history of intermittent joint pain, fleeting rashes, and low-grade fever. She has a family history of type 2 diabetes mellitus and Hashimoto thyroiditis. She reports moderate depression, but the review of systems is otherwise negative. On examination, she has multiple trigger points in the trapezius muscle and mild loss of rotation of the cervical spine. Her joint and neurovascular findings are normal. Testing reveals a 1:40 antinuclear antibody (ANA) titer and a weakly positive rheumatoid factor (RF) titer of 22 IU per mL. What diagnostic tests, if any, should be ordered next?
Testing for Connective Tissue Disorders
The hallmark of a connective tissue disorder is synovitis, which may be accompanied by other features such as the Raynaud phenomenon, serositis, nephritis, or decreased platelet or leucocyte count. Although synovitis is common to all connective tissue disorders, there are specific features and serologic test results that characterize each one (Table 1).1,2
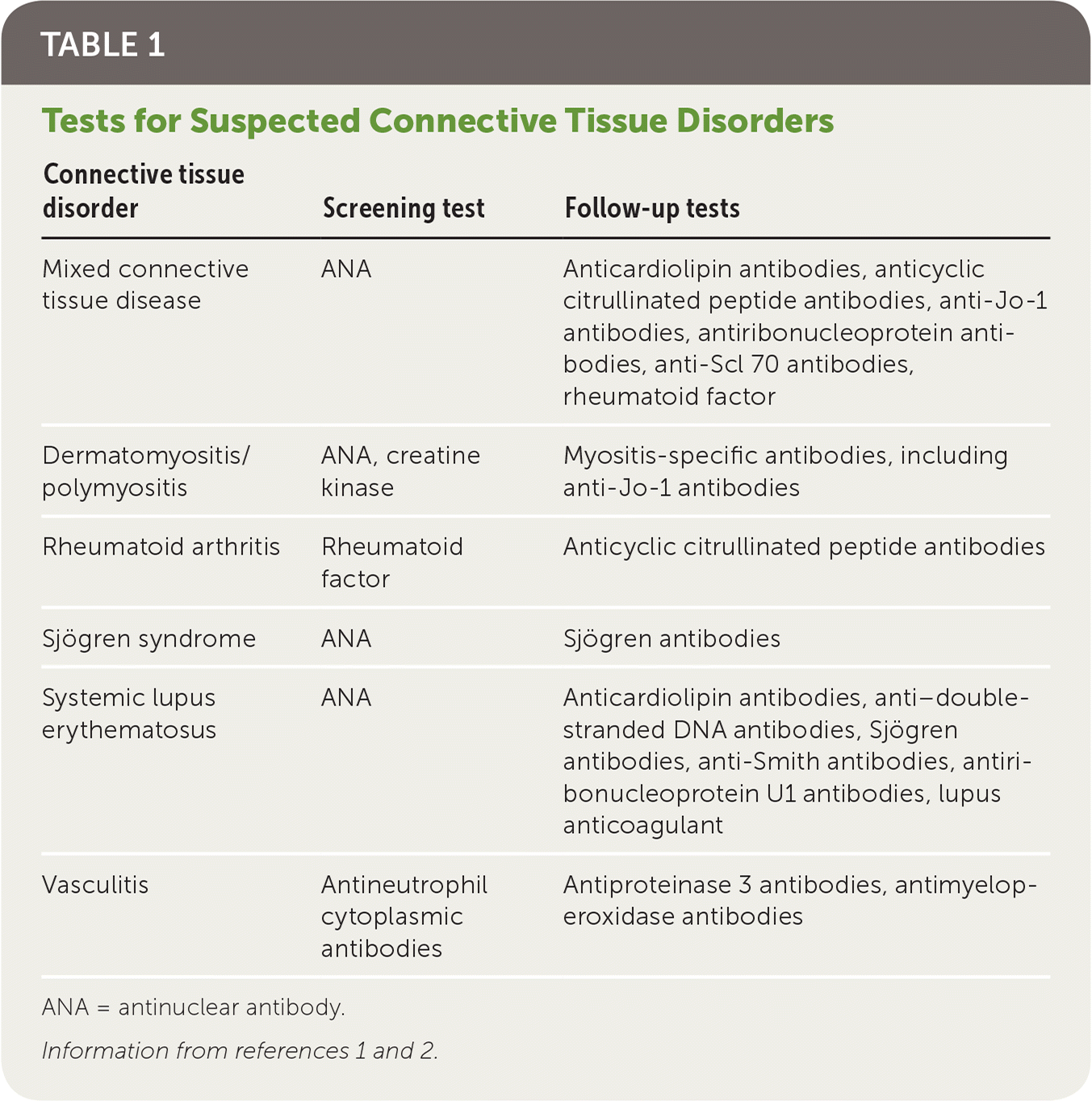
| Connective tissue disorder | Screening test | Follow-up tests |
|---|---|---|
| Mixed connective tissue disease | ANA | Anticardiolipin antibodies, anticyclic citrullinated peptide antibodies, anti-Jo-1 antibodies, antiribonucleoprotein antibodies, anti-Scl 70 antibodies, rheumatoid factor |
| Dermatomyositis/polymyositis | ANA, creatine kinase | Myositis-specific antibodies, including anti-Jo-1 antibodies |
| Rheumatoid arthritis | Rheumatoid factor | Anticyclic citrullinated peptide antibodies |
| Sjögren syndrome | ANA | Sjögren antibodies |
| Systemic lupus erythematosus | ANA | Anticardiolipin antibodies, anti–double-stranded DNA antibodies, Sjögren antibodies, anti-Smith antibodies, antiribonucleoprotein U1 antibodies, lupus anticoagulant |
| Vasculitis | Antineutrophil cytoplasmic antibodies | Antiproteinase 3 antibodies, antimyeloperoxidase antibodies |
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is the prototypic autoimmune disease characterized by production of autoantibodies resulting in end-organ inflammation. The diagnosis is made on the basis of clinical features and serologic test results.
SLE primarily affects women and often starts in those of childbearing age. It should be suspected in patients with arthritis; mucositis; and renal, hematologic, or central nervous system involvement. The hallmark of SLE is the presence of ANA, which is found in more than 95% of affected patients.3 The likelihood of SLE is low in patients with negative ANA titers who do not have the full constellation of symptoms (e.g., only joint pain and rash).
In addition to ANA testing, a complete metabolic panel can be ordered to evaluate renal and hepatic function; a complete blood count with differential can help screen for lymphopenia, thrombocytopenia, and anemia; and urinalysis with microscopy can assess for hematuria, pyuria, and proteinuria.
ANTINUCLEAR ANTIBODIES
ANA is an antibody against a nuclear component of a cell. Although nearly all patients with SLE have positive ANA titers, most patients with a positive titer do not have SLE.4
The most accurate test for ANA is via indirect immunofluorescence assay using human epithelial cells, which act as a substrate for the antibody. The sensitivity of ANA detected via indirect immunofluorescence testing is 93%, and the specificity is 57%.5 The positive likelihood ratio (LR+) for the diagnosis of SLE is 2.2, and the negative likelihood ratio (LR–) is 0.1.5
Because indirect immunofluorescence is labor intensive, many commercial laboratories are moving to enzyme-linked immunosorbent assay, which is less expensive but less accurate.6 The sensitivity and specificity of ANA testing via this method are 81.9% and 79.6%, respectively; the LR+ is 2.97, and LR– is 0.25.7
ANA results are reported using a titer, such as 1:320. Generally, the higher the titer, the more likely the patient is to have a connective tissue disorder. The titer shows how many times the patient's serum was diluted before the antibodies could no longer be detected. Thus, a titer of 1:640 shows a greater concentration of ANA than 1:40. Once a patient has a positive ANA titer, it is rarely helpful to repeat the test; ANA levels fluctuate and do not reflect disease activity.
A positive ANA titer can occur in other connective tissue disorders, such as Sjögren syndrome and scleroderma; therefore, it cannot be used to definitively diagnose SLE. In SLE, the ANA result will commonly have a homogeneous or rim pattern. In Sjögren syndrome there will often be a speckled pattern; in scleroderma there will be a nucleolar pattern; and in limited scleroderma (i.e., CREST syndrome [calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia]) there will be a pattern of centromere staining.
ANA titers can be falsely positive in many diseases that are characterized by autoimmunity and nonspecific antibody production. These include Hashimoto thyroiditis, autoimmune liver disease, viral infections such as hepatitis C and human immunodeficiency virus infections, some cancers, pulmonary fibrosis, and type 1 diabetes mellitus. Thus, when ANA titers are positive and suspicion remains for SLE or another connective tissue disorder, more specific assays should be performed to detect other antigens to nuclear components.8 Testing for the presence of anti– double-stranded DNA antibodies is advised,9 as is testing for anti-Smith antibodies, antiribonucleoprotein antibodies, Sjögren antibodies (anti-SS-A and anti-SS-B), anticardiolipin, and lupus anticoagulant.
ANTI–DOUBLE-STRANDED DNA ANTIBODIES
Anti–double-stranded DNA antibodies are a hallmark of SLE. Their sensitivity is 70%, and specificity is 95%.10 This test can also be useful for disease monitoring because, in some patients, these antibodies are deposited in and cause damage to the kidneys. Nevertheless, there are many patients in whom an increase in anti–double-stranded DNA antibody levels does not correlate with disease.11 Testing may be helpful if the pretest probability for SLE is high or when monitoring for a possible flare, because antibody levels can increase in a subset of patients with active nephritis.12
ANTI-SMITH ANTIBODIES
Anti-Smith antibodies have the greatest specificity for SLE (98.6%) and are included in the diagnostic criteria for SLE.13 However, their sensitivity is low (39.7%). Therefore, a positive test result is essentially diagnostic of SLE, but a negative result does not exclude it.
Drug-Induced Lupus
Drug-induced lupus should be considered in patients who are taking medications such as procainamide, hydralazine, beta blockers, or phenytoin (Dilantin) when they develop arthralgia, hematologic abnormalities, rash, or serositis.14 Life-threatening disease is rare, and symptoms often resolve when the medication is discontinued.
Antihistone antibodies are classically associated with drug-induced lupus, although they may be present in other conditions such as SLE, auto-immune hepatitis, juvenile idiopathic arthritis, myositis, and scleroderma. An ANA titer should be obtained if a patient develops features of SLE while taking one of the medications listed above; if the results are positive, follow-up testing for antihistone antibodies is recommended.11 The sensitivity of antihistone antibodies for drug-induced lupus is 95%, and specificity exceeds 90%.15
Sjögren Syndrome
Sjögren syndrome results from lymphocytic infiltration of exocrine glands, classically the salivary and tear glands. The clinical hallmark is dryness of the mouth and eyes (xerostomia and sicca). Because dry mouth and eyes are also common symptoms in other conditions, serologic testing can be helpful to identify patients with Sjögren syndrome.
Diagnostic criteria include the presence of ocular signs and symptoms, abnormal salivary gland pathology, and positive autoantibody titers, including ANA. The sensitivity and specificity of a positive ANA result are 48% and 52%, respectively; the LR+ is 0.99, and the LR– is 1.01.11
Sjögren antibodies may also be present when the syndrome develops in patients with other connective tissue disorders. For example, when these antibodies are detected in patients with rheumatoid arthritis, the risk of secondary Sjögren syndrome is increased. When present in patients with SLE, the risk of cytopenias, subacute cutaneous SLE, and nephritis is increased.16 Anti-SS-A can cross the placenta and is associated with neonatal complications. The risk of complete heart block in newborns is about 2% in the first pregnancy of women with an underlying connective tissue disorder.17
Mixed Connective Tissue Disease
Mixed connective tissue disease is an overlap syndrome of SLE, myositis, and scleroderma. Patients classically present with the Raynaud phenomenon, pulmonary hypertension, arthritis, and myositis.
When mixed connective tissue disease is suspected, an ANA titer is the best initial screening test; if results are positive, further testing should include antiribonucleoprotein antibodies. Although these antibodies are present in several connective tissue disorders, their sensitivity for diagnosing mixed connective tissue disease is 71% to 100%, and the specificity is 84% to 100%.18
Scleroderma
Scleroderma is a clinical syndrome characterized by tight skin, interstitial lung disease, pulmonary hypertension, and diffuse organ fibrosis. Although this condition is rare, antibody testing can help with the diagnosis. Initial testing should include an ANA titer; if results are positive, further testing should include anticentromere and anti-Scl 70 antibodies, which are present in patients with limited and diffuse scleroderma, respectively. The presence of anti-Scl 70 antibodies is associated with increased mortality and a greater incidence of interstitial lung disease.19 The sensitivity and specificity of anti-Scl 70 antibodies by enzyme-linked immunosorbent assay are 43% and 100%, respectively.20
Dermatomyositis and Polymyositis
The inflammatory muscle diseases dermatomyositis and polymyositis should be suspected in patients who have muscle weakness with elevated levels of muscle enzymes such as creatine kinase, myopathic changes on electromyography, and characteristic muscle pathology.
Approximately 80% of patients with dermatomyositis or polymyositis have a positive ANA titer.21 One-third have antisynthetase syndrome, a condition that includes nonerosive arthritis, fever, the Raynaud phenomenon, interstitial lung disease, and “mechanic's hands” (fissuring on the distal fingertips). Myositis-specific antibodies are present in only about 20% of patients with this syndrome.21 Anti-Jo-1 antibodies are directed against histidyl-transfer RNA synthetase and comprise 80% of the myositis-specific antibodies in antisynthetase syndrome.21 Because of their low prevalence, these antibodies should not be measured routinely in patients with myalgias.
Rheumatoid Arthritis
RHEUMATOID FACTOR
Rheumatoid arthritis is a symmetric small-joint arthropathy affecting the hands, wrists, and feet and is associated with joint pain and morning stiffness. Autoantibody testing can be helpful in establishing the diagnosis.
RF is an autoantibody produced from polyclonal β cell activation. Testing is typically for immunoglobulin M RF. A positive RF titer in a patient with joint pain increases the probability of rheumatoid arthritis. The higher the titer, the more likely the patient will have erosive joint disease, extra-articular manifestations, and a poor outcome.22
However, a positive RF titer does not provide a definitive diagnosis of rheumatoid arthritis. The sensitivity and specificity are 69% and 85%, respectively; the LR+ is 4.86, and the LR– is 0.38.23 Thus, RF should not be used indiscriminately as a screening test in patients with joint pain24 because many conditions can stimulate β cells to produce antibodies, including viral infections, endocarditis, lymphoma, and cryoglobulinemia (Table 2).25

| Connective tissue disorder (rate of positive titer) | |
| Cryoglobulinemia (40% to 100%) | |
| Mixed connective tissue disease (50% to 60%) | |
| Rheumatoid arthritis (50% to 90%) | |
| Sjögren syndrome (75% to 95%) | |
| Systemic lupus erythematosus (15% to 35%) | |
| Systemic sclerosis (20% to 30%) | |
| Nonrheumatic conditions | |
| Aging | |
| Infections | |
| Bacterial endocarditis | |
| Hepatitis | |
| Parasitic diseases | |
| Syphilis | |
| Tuberculosis | |
| Viral infections (especially mumps, rubella, and influenza) | |
| Pulmonary diseases | |
| Asbestosis | |
| Interstitial pulmonary fibrosis | |
| Sarcoidosis | |
| Silicosis | |
| Other conditions | |
| Cancers (especially leukemia and colon cancers) | |
| Primary biliary cirrhosis | |
ANTICYCLIC CITRULLINATED PEPTIDE ANTIBODIES
Because of the low sensitivity and specificity of RF for rheumatoid arthritis, other tests have been developed. An enzyme-linked immunosorbent assay should be considered to detect anticyclic citrullinated peptide antibodies in patients with a moderate clinical pretest probability of rheumatoid arthritis. Its pooled sensitivity and specificity are 67% and 95%, respectively; the LR+ is 12.46, and the LR– is 0.36.26
Granulomatosis with Polyangiitis
Granulomatosis with polyangiitis, formerly known as Wegener granulomatosis, is a rare disease characterized by necrotizing vasculitis in small and medium blood vessels. Patients can present with symptoms such as recurrent sinusitis, epistaxis, airway inflammation, neuropathy, and glomerulonephritis.
ANTINEUTROPHIL CYTOPLASMIC ANTIBODIES
Although a histologic biopsy showing vasculitis is the preferred diagnostic test for granulomatosis with polyangiitis, antineutrophil cytoplasmic antibody (ANCA) testing has clinical utility as a diagnostic marker. ANCAs are antibodies directed against granules in the neutrophil cytoplasm.
The sensitivity of ANCA testing for granulomatosis with polyangiitis is 66%, and the specificity is 98%.28 However, because the prevalence of vasculitis in the general population is low, a positive ANCA result is often a false positive. Thus, ANCA testing can help diagnose granulomatosis with polyangiitis only when the pretest probability is high. ANCA testing should be ordered for patients with pulmonary-renal syndrome, rapidly progressive renal failure, mononeuritis multiplex, or pulmonary hemorrhage. It should not be used in patients with routine sinusitis unless some features of systemic vasculitis are present.29,30
Other Tests
HUMAN LEUKOCYTE B27 ANTIGEN
The HLA-B27 gene is the hallmark of ankylosing spondylitis and is present in about 95% of persons with the disease.31 However, it is also present in those with other seronegative spondyloarthropathies, such as reactive arthritis, psoriatic arthritis, and enteropathic arthritis. HLA-B27 is also present in up to 6% of healthy persons in the United States32; therefore, testing should not be performed routinely in patients with back pain. HLA-B27 antigen testing is most useful when an inflammatory disorder of the back, joints, chest, or eyes is suspected, or when further evidence is needed to help confirm a suspected diagnosis of ankylosing spondylitis.
ERYTHROCYTE SEDIMENTATION RATE
The erythrocyte sedimentation rate (ESR) is a crude measure of inflammation. It assesses the vertical distance a column of blood falls in one hour in an anticoagulated Westergren tube. ESR elevation occurs in many conditions, including polymyalgia rheumatica and temporal arteritis; the ESR can be helpful in monitoring disease activity in those conditions.
Elevated ESR is one of the American College of Rheumatology classification criteria for polymyalgia rheumatica.33 It has a sensitivity of 80% for polymyalgia rheumatica and 95% for temporal arteritis.34 The ESR generally increases with age because of changes in fibrinogen levels that affect the fall rate of red blood cells during testing. A normal ESR in a man is generally less than age÷2, and in a woman is less than (age+10)÷2. Table 3 outlines factors that can influence the ESR.35

| Increase erythrocyte sedimentation rate |
| Anemia |
| Elevated fibrinogen level: infection, inflammation, malignancy |
| Female sex |
| Macrocytosis |
| Older age |
| Pregnancy |
| Technical factors: dilution problem, increased specimen temperature, tilted tube |
| Decrease erythrocyte sedimentation rate |
| Acanthocytosis (crenated red blood cells) |
| Anisocytosis (red blood cells of unequal size) |
| Extreme leukocytosis |
| Microcytosis |
| Polycythemia |
| Protein abnormalities: hypofibrinogenemia, hypogammaglobulinemia, dysproteinemia with hyperviscosity |
| Sickle cell disease |
| Spherocytosis |
| Technical factors: dilutional problem, inadequate mixing, clotting of blood sample, short tube, vibration during testing |
The ESR has also been used as a “sickness index” to screen for patients with underlying systemic disease.36 However, the benefit of such screening has not been supported by research.
C-REACTIVE PROTEIN
C-reactive protein (CRP) is more sensitive than ESR for detecting inflammation. It is produced in the liver and correlates better than ESR with disease activity.37 An increase in CRP level occurs much earlier than with other acute-phase reactants, usually four to six hours after tissue injury.
CRP testing is appropriate if the pretest probability of a connective tissue disorder is moderate or high based on clinical presentation and the ESR is normal. High-sensitivity CRP testing is more precise but should be reserved for cardiovascular risk assessment.38
Final Comment
The patient described in the clinical scenario does not have typical symptoms of a connective tissue disorder. Instead, she has classic myofascial pain with trapezius trigger points and weakly positive ANA and RF titers. Because her pre-test probability of a connective tissue disorder is low, the indication for ordering these tests and the significance of their results are uncertain. The patient's family history of Hashimoto thyroiditis increases her risk of false-positive autoantibody test results, and she has no clinical evidence of a connective tissue disorder. Therefore, no further immuno-logic tests are warranted.
Tests for connective tissue disorders should be performed selectively in the correct clinical context to avoid incorrect diagnoses and unnecessary costs, which can vary from approximately $15 for an RF titer39 to many hundreds of dollars for a multitest panel. Physicians should avoid using a shotgun approach to diagnostic testing and should limit tests to those necessary to confirm a specific clinically suspected condition.
This article updates a previous article on this topic by Lane and Gravel.40
Data Sources: A PubMed search was completed in Clinical Queries using the key terms autoantibodies, antinuclear antibody, rheumatoid factor, and erythrocyte sedimentation rate. The search included meta-analyses and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, and the National Guideline Clearinghouse database. Search dates: January 15, 2017, to March 28, 2018.