Am Fam Physician. 2018;98(5):online
Author disclosure: No relevant financial affiliations.

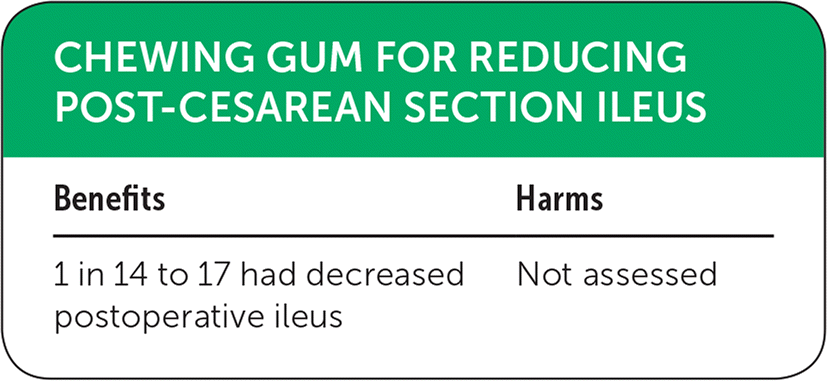
| Benefits | Harms |
|---|---|
| 1 in 14 to 17 had decreased postoperative ileus | Not assessed |
Details for This Review
Study Population: More than 3,000 women (17 trials) who had just had cesarean section, mostly from the Middle East and Asia
Efficacy End Points: Primary: postoperative ileus, time to flatus; secondary: length of stay, time to defecation, need for enema, need for antiemetic, patient satisfaction
Harm End Points: Not assessed
Narrative: Postoperative ileus complicates a significant percentage of surgeries. By delaying the normal return to gastrointestinal function, postoperative ileus may increase patient discomfort and has been shown to prolong hospitalization by five days, increasing total costs by almost $1.5 billion annually in the United States.1 Surgeons and medical teams have tried numerous pharmacologic and nonpharmacologic interventions for postoperative ileus. The perfect intervention would be physiologic, effective, safe, and inexpensive. Early reintroduction of diet, although ideal, is not tolerated by some patients. Chewing gum may trigger salivation and the same neurodigestive processes that lead to normal gastrointestinal function and could represent a viable alternative to early diet reintroduction. Other research has shown gum chewing can reduce time to flatus, time to defecation, length of hospital stay, and the time to tolerate a diet in postoperative gynecologic oncology patients.2
Two studies examined the effectiveness of chewing gum to reduce post-cesarean section ileus.3,4 One review included 17 randomized controlled trials with more than 3,000 patients, and examined time to flatus and rate of ileus as primary outcomes.3 For time to flatus, they found 13 studies including 2,399 women. On average, chewing gum reduced this metric by seven hours. Regarding rate of ileus, four studies with 1,139 women demonstrated a reduction in incidence from 11% to 5%, yielding a number needed to treat (NNT) of 17. Secondary outcomes showed a reduction in time to defecation, as well as duration of hospital stay. The need for pain control or antiemetics did not differ between intervention and control groups.3
The other review focused on time to flatus as the primary outcome.4 Among 2,459 women, time to flatus was reduced from 29.5 to 23.1 hours. Secondary outcomes demonstrated reductions in rate of ileus (11.4% to 4.6%), time to bowel sounds, time to defecation, time to hunger, as well as decreased nausea and vomiting episodes and higher patient satisfaction.
An earlier source study found a similar reduction in rate of ileus (7.4%, NNT = 14), time to flatus, and time to defecation.5 The study also found a significant decrease in need for antiemetics and pain medication, but without an effect on length of stay.
None of the meta-analyses were able to truly assess harms because these were not reported in the included trials. Chewing gum did appear to be well tolerated with only three of 925 women discontinuing treatment. Given the well-established safety of chewing gum, true adverse events are likely insubstantial. Theoretical harms include choking, dental pain/temporomandibular dysfunction, and distaste. A separate study of patients who underwent minimally invasive gynecologic surgery demonstrated no related adverse effects.6
Caveats: Overall, our update focused on two meta-analyses that included studies of fairly low quality. This was limited by a general inability to blind patients to chewing gum, but a number of studies also lacked blinding of observers, and certain trials had incomplete concealment.
Additionally, the authors report standardized mean differences for most of the outcomes. Although this allows for combining studies using different outcome measures, it also requires study populations to be similar. Given the heterogeneity in the studies, this may not be true. Heterogeneity was largely due to differences in patient populations, specific interventions, variations in diet, and outcome definitions. Included source trials varied in the nature of the intervention (e.g., when introduced, for how long each day, total duration) and in specifics of the outcome (i.e., definition of ileus). The validity of one of the trials was further limited by the use of multiple primary outcomes.3
Most of the included trials were conducted at single centers in low-income countries across the Middle East and Asia. Therefore, the external validity in the United States, Europe, and other more developed countries is uncertain. However, there is no reason to believe demographic differences would affect the proposed mechanism of action of chewing gum in this population or limit the generalizability of the results.
In the end, all of the studies suggest consistently significant improvements in the less patient-oriented outcomes (rate of ileus, time to flatus).3–5,7 The more patient-oriented outcomes have been inconsistently demonstrated. Length of hospital stay was found to be increased in some trials,3,7 but decreased in at least one other study.5 Similarly, nausea and vomiting have been reported to be increased in some studies,4,5 and decreased in others.3 Although the current body of literature suggests a need for higher-quality, more robust research, the simplicity, frugality, and safety of chewing gum makes it a viable option to offer women undergoing cesarean section.
Copyright © 2018 MD Aware, LLC (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Assistant Medical Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/chewing-gum-reducing-post-cesarean-section-ileus/.