
Am Fam Physician. 2018;98(7):413-416
Author disclosure: In 2016, Dr. Andrews received compensation for travel expenses to attend a meeting on hepatitis B from Gilead Sciences, Inc., which manufactures drugs used to treat hepatitis C. Dr. Andrews declined an honorarium from Gilead.
Hepatitis C virus (HCV) causes the only chronic viral disease that is consistently curable with medication. However, it causes more deaths in the United States than the next 60 reportable infections combined, including human immunodeficiency virus (HIV), tuberculosis, and pneumococcal disease.1
The epidemiology of HCV infection is complex and differs in key ways from hepatitis B and HIV infections. HCV is not commonly transmitted through sex, but it is far more infectious than hepatitis B virus and HIV when transmitted via injection and intranasal drug use.2 Despite effective treatments, the incidence of HCV infection is increasing in the United States. High-risk groups include persons born between 1945 and 1965, those who use certain illicit drugs, and those who are incarcerated.2
Sustained viral response (SVR), defined as the persistent absence of detectable virus in the blood, is the goal of hepatitis C treatment. The term SVR12 is used when SVR occurs 12 weeks or more after completion of antiviral treatment. SVR12 is widely accepted as reflecting the eradication of HCV from the body, as opposed to mere suppression. Medications can eliminate HCV because the virus does not have a hidden reservoir in the body, unlike hepatitis B virus.3
SVR was first achieved in 1986 using interferon; ribavirin (Virazole) was added in later regimens. Complex treatment protocols often caused severe adverse effects and were less effective against certain viral genotypes.4 Currently recommended treatments use direct-acting antivirals. When used for the specified durations, these medications consistently achieve SVR12 rates at or above 90%, and the newest agents are effective against all HCV genotypes 5–10 (Table 111 ).
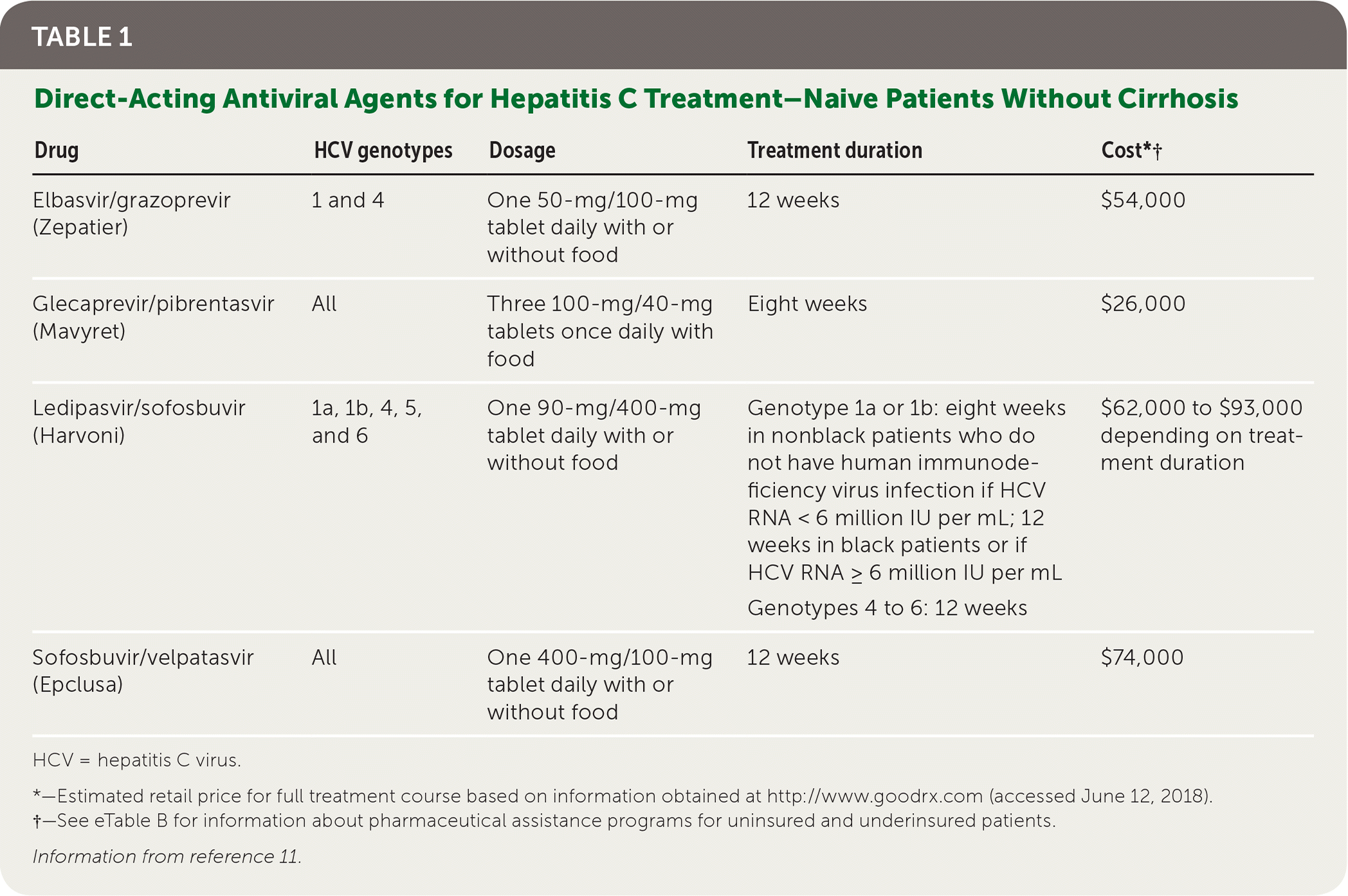
| Drug | HCV genotypes | Dosage | Treatment duration | Cost*† |
|---|---|---|---|---|
| Elbasvir/grazoprevir (Zepatier) | 1 and 4 | One 50-mg/100-mg tablet daily with or without food | 12 weeks | $54,000 |
| Glecaprevir/pibrentasvir (Mavyret) | All | Three 100-mg/40-mg tablets once daily with food | Eight weeks | $26,000 |
| Ledipasvir/sofosbuvir (Harvoni) | 1a, 1b, 4, 5, and 6 | One 90-mg/400-mg tablet daily with or without food | Genotype 1a or 1b: eight weeks in nonblack patients who do not have human immunodeficiency virus infection if HCV RNA < 6 million IU per mL; 12 weeks in black patients or if HCV RNA ≥ 6 million IU per mL Genotypes 4 to 6: 12 weeks | $62,000 to $93,000 depending on treatment duration |
| Sofosbuvir/velpatasvir (Epclusa) | All | One 400-mg/100-mg tablet daily with or without food | 12 weeks | $74,000 |
SVR can reverse liver fibrosis, and even cirrhosis can start to reverse after viral hepatitis is cured.12 It is not known whether SVR achieved with direct-acting antivirals reduces the occurrence of hepatocellular carcinoma, but recent evidence is encouraging.13,14 Reflecting the systemic nature of chronic hepatitis C, persons who achieve SVR have a lower incidence of diabetes mellitus, glomerulonephritis, non-Hodgkin lymphoma, stroke, and other conditions.15
It is not clear whether the high cost of direct-acting antivirals is offset by long-term savings related to reduced morbidity and mortality from hepatitis C. Economic estimates vary by health system and by type of study analysis (e.g., whether the study end point is focused on achieving SVR or on post-SVR improvements in HCV-related extrahepatic disease).16–19 A typical treatment course is one to three pills per day for eight to 12 weeks, and is usually well tolerated. Viral counts are obtained at four weeks to assess effectiveness. A final test is performed 12 weeks or more after the last pill is taken. Guidelines recommend that patients with pretreatment evidence of cirrhosis have lifelong surveillance for hepatocellular carcinoma using liver imaging and α-fetoprotein testing at six-month intervals.20 Prior HCV infection and treatment do not confer immunity, so patients at high risk of reinfection (e.g., those with active injection or intranasal drug use, sex partners of infected persons) should be screened periodically.21
The Centers for Disease Control and Prevention encourages family physicians to treat hepatitis C.22 Outcomes when primary care physicians prescribe direct-acting antivirals to patients with uncomplicated hepatitis C are comparable to those of subspecialists.23 Patients with hepatitis C who do not have cirrhosis, or who have compensated cirrhosis, typically respond well to management by primary care physicians. Treatment in patients with decompensated cirrhosis (characterized by the presence of ascites, esophageal varices, or hepatic encephalopathy) is complex. Family physicians should be able to identify these patients and refer them to a gastroenterologist or hepatologist.
Training and support are available for family physicians who wish to treat patients with hepatitis C. A reasonable initial approach would be to complete one or more self-paced online study courses (eTable A). Because some insurers require consultation with a subspecialist before they will cover a direct-acting antiviral, use of a telehealth program such as Project ECHO (Extension for Community Healthcare Outcomes) can be helpful. These resources provide real-time affordable access to subspecialist consultation using a case presentation format.
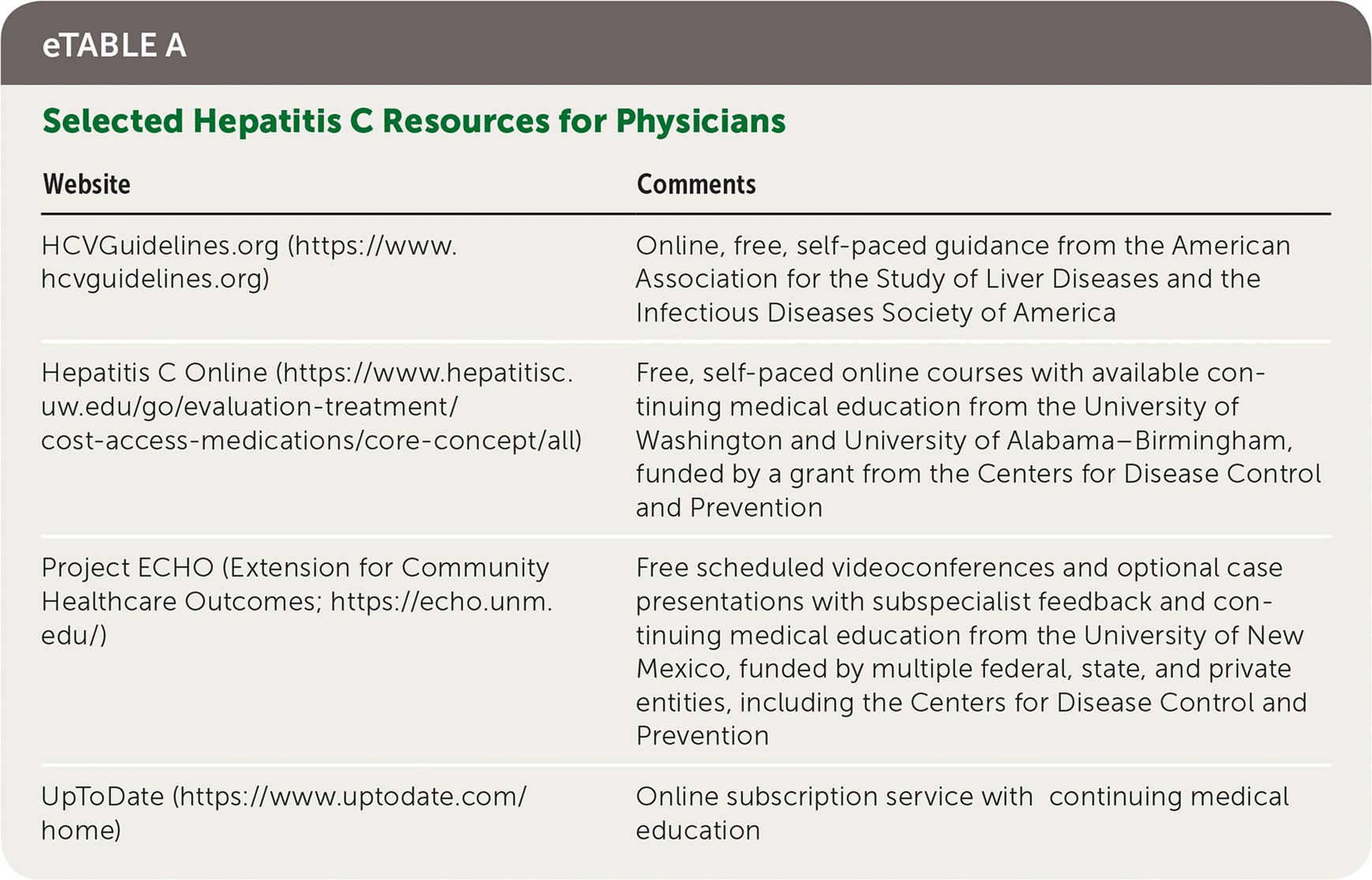
| Website | Comments |
|---|---|
| HCVGuidelines.org (https://www.hcvguidelines.org) | Online, free, self-paced guidance from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America |
| Hepatitis C Online (https://www.hepatitisc.uw.edu/go/evaluation-treatment/cost-access-medications/core-concept/all) | Free, self-paced online courses with available continuing medical education from the University of Washington and University of Alabama–Birmingham, funded by a grant from the Centers for Disease Control and Prevention |
| Project ECHO (Extension for Community HealthcareOutcomes; https://echo.unm.edu/) | Free scheduled videoconferences and optional case presentations with subspecialist feedback and continuing medical education from the University of New Mexico, funded by multiple federal, state, and private entities, including the Centers for Disease Control and Prevention |
| UpToDate (https://www.uptodate.com/home) | Online subscription service with continuing medical education |
By reducing the prevalence of HCV infection, direct-acting antivirals are a key tool in the treatment-as-prevention approach.24 They are a necessary—but not sufficient—component of any worldwide program to eliminate HCV. As with many treatments, these medications are most effective when combined with evidence-based public health measures, such as accessibility of clean needles in exchange for used needles among persons who inject drugs.25
Although the new direct-acting antivirals to treat hepatitis C are expensive, especially in the United States, there are multiple pharmaceutical assistance programs to ensure access to therapy for all patients, regardless of their ability to pay (eTable B). By participating in treatment programs and advocating for effective policy decisions, such as needle exchanges, family physicians can have a major impact on the hepatitis C epidemic.
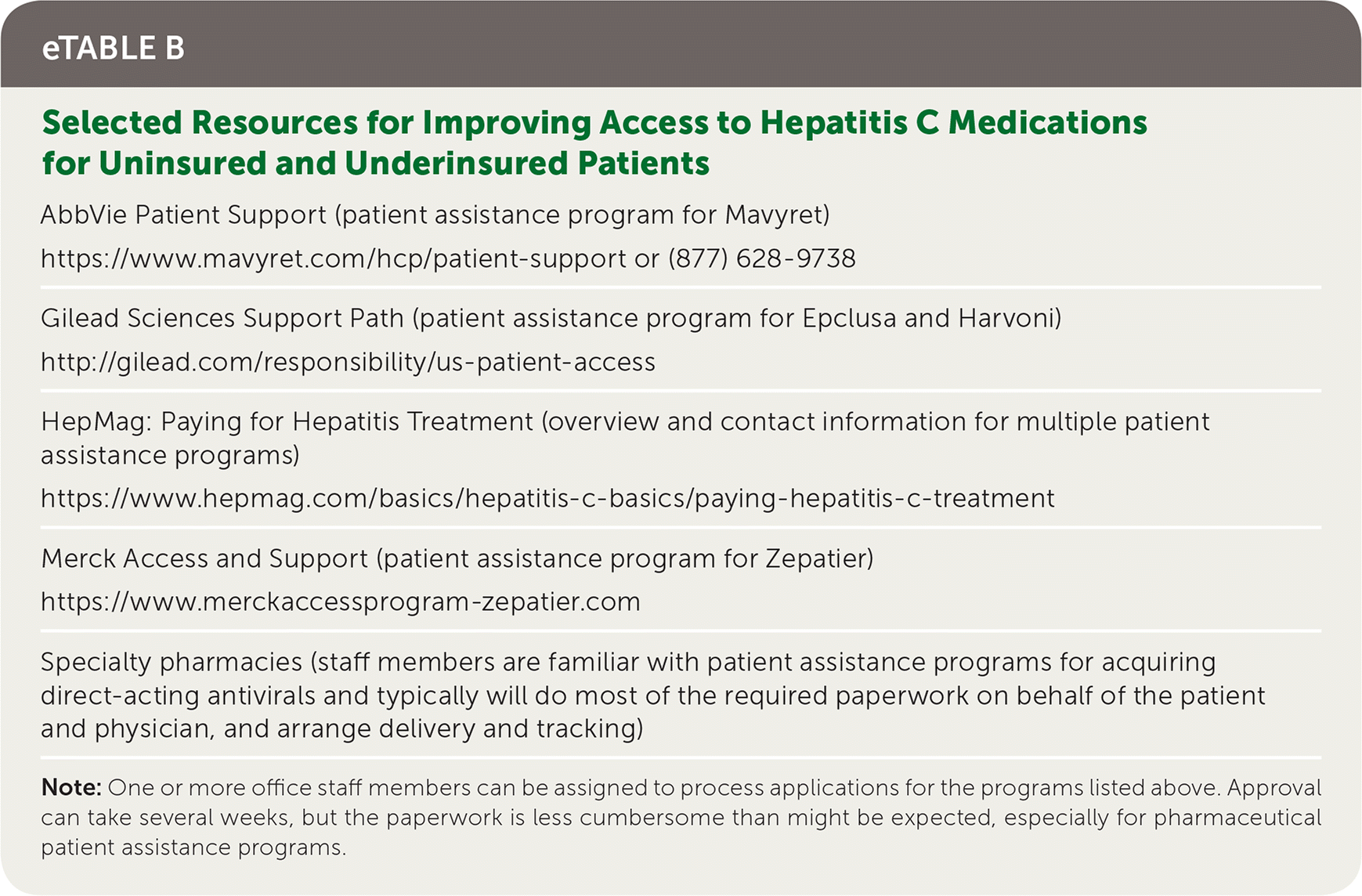
AbbVie Patient Support (patient assistance program for Mavyret)
|
| Gilead Sciences Support Path (patient assistance program for Epclusa and Harvoni) |
| HepMag: Paying for Hepatitis Treatment (overview and contact information for multiple patient assistance programs) |
| Merck Access and Support (patient assistance program for Zepatier) |
| Specialty pharmacies (staff members are familiar with patient assistance programs for acquiring direct-acting antivirals and typically will do most of the required paperwork on behalf of the patient and physician, and arrange delivery and tracking) |
