
Am Fam Physician. 2018;98(8):online
Related editorial: Counseling Patients About Prostate Cancer Screening.
As published by the U.S. Preventive Services Task Force.
Summary of Recommendations and Evidence
For men aged 55 to 69 years, the decision to undergo periodic prostate-specific antigen (PSA)–based screening for prostate cancer should be an individual one. Before deciding whether to be screened, men should have an opportunity to discuss the potential benefits and harms of screening with their clinician and to incorporate their values and preferences in the decision. Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results that require additional testing and possible prostate biopsy; overdiagnosis and overtreatment; and treatment complications, such as incontinence and erectile dysfunction. In determining whether this service is appropriate in individual cases, patients and clinicians should consider the balance of benefits and harms on the basis of family history, race/ethnicity, comorbid medical conditions, patient values about the benefits and harms of screening and treatment-specific outcomes, and other health needs. Clinicians should not screen men who do not express a preference for screening (Table 1). C recommendation.
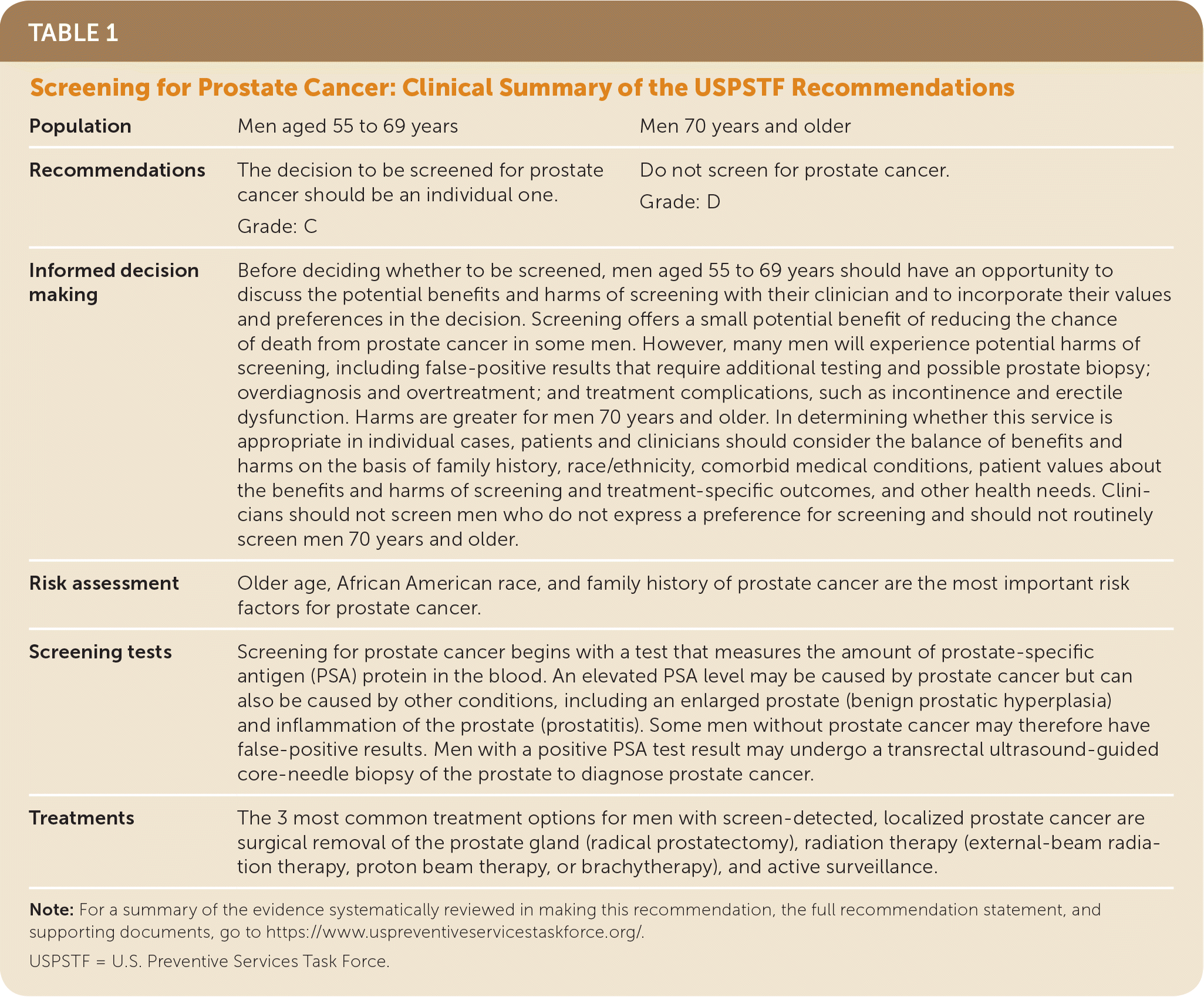
| Population | Men aged 55 to 69 years | Men 70 years and older |
| Recommendations | The decision to be screened for prostatecancer should be an individual one. Grade: C | Do not screen for prostate cancer. Grade: D |
| Informed decision making | Before deciding whether to be screened, men aged 55 to 69 years should have an opportunity to discuss the potential benefits and harms of screening with their clinician and to incorporate their values and preferences in the decision. Screening offers a small potential benefit of reducing the chance of death from prostate cancer in some men. However, many men will experience potential harms of screening, including false-positive results that require additional testing and possible prostate biopsy; overdiagnosis and overtreatment; and treatment complications, such as incontinence and erectile dysfunction. Harms are greater for men 70 years and older. In determining whether this service is appropriate in individual cases, patients and clinicians should consider the balance of benefits and harms on the basis of family history, race/ethnicity, comorbid medical conditions, patient values about the benefits and harms of screening and treatment-specific outcomes, and other health needs. Clinicians should not screen men who do not express a preference for screening and should not routinely screen men 70 years and older. | |
| Risk assessment | Older age, African American race, and family history of prostate cancer are the most important risk factors for prostate cancer. | |
| Screening tests | Screening for prostate cancer begins with a test that measures the amount of prostate-specific antigen (PSA) protein in the blood. An elevated PSA level may be caused by prostate cancer but can also be caused by other conditions, including an enlarged prostate (benign prostatic hyperplasia) and inflammation of the prostate (prostatitis). Some men without prostate cancer may therefore have false-positive results. Men with a positive PSA test result may undergo a transrectal ultrasound-guided core-needle biopsy of the prostate to diagnose prostate cancer. | |
| Treatments | The 3 most common treatment options for men with screen-detected, localized prostate cancer are surgical removal of the prostate gland (radical prostatectomy), radiation therapy (external-beam radiation therapy, proton beam therapy, or brachytherapy), and active surveillance. | |
The USPSTF recommends against PSA-based screening for prostate cancer in men 70 years and older. D recommendation.
See the Clinical Considerations section for more information on screening higher risk populations, including African American men and men with a family history of prostate cancer.
Rationale
IMPORTANCE
Prostate cancer is one of the most common types of cancer that affects men. In the United States, the lifetime risk of being diagnosed with prostate cancer is approximately 13%, and the lifetime risk of dying of prostate cancer is 2.5%.1 Many men with prostate cancer never experience symptoms and, without screening, would never know they have the disease. In autopsy studies of men who died of other causes, more than 20% of men aged 50 to 59 years and more than 33% of men aged 70 to 79 years were found to have prostate cancer.2 In some men, the cancer is more aggressive and leads to death. The median age of death from prostate cancer is 80 years, and more than two-thirds of all men who die of prostate cancer are older than 75 years.1 African American men have an increased lifetime risk of prostate cancer death compared with those of other races/ethnicities (4.2% for African American men, 2.9% for Hispanic men, 2.3% for white men, and 2.1% for Asian and Pacific Islander men).1
DETECTION
Screening for prostate cancer begins with a test that measures the amount of PSA protein in the blood. An elevated PSA level may be caused by prostate cancer but can also be caused by other conditions, including an enlarged prostate (benign prostatic hyperplasia) and inflammation of the prostate (prostatitis). Some men without prostate cancer may therefore have positive screening results (i.e., false-positive results). Men with a positive PSA test result may undergo a transrectal ultrasound-guided core-needle biopsy of the prostate to diagnose prostate cancer.
BENEFITS OF EARLY DETECTION AND TREATMENT
The goal of screening for prostate cancer is to identify high-risk, localized prostate cancer that can be successfully treated, thereby preventing the morbidity and mortality associated with advanced or metastatic prostate cancer.
Adequate evidence from randomized clinical trials (RCTs) shows that PSA-based screening programs in men aged 55 to 69 years may prevent approximately 1.3 deaths from prostate cancer over approximately 13 years per 1,000 men screened.3,4 Screening programs may also prevent approximately 3 cases of metastatic prostate cancer per 1,000 men screened.3 Current results from screening trials show no reductions in all-cause mortality from screening. There is inadequate evidence to assess whether the benefits for African American men and men with a family history of prostate cancer aged 55 to 69 years are different from the benefits for the average-risk population. There is also inadequate evidence to assess whether there are benefits to starting screening in these high-risk groups before age 55 years.
Adequate evidence from RCTs is consistent with no benefit of PSA-based screening for prostate cancer on prostate cancer mortality in men 70 years and older.
HARMS OF EARLY DETECTION AND TREATMENT
The harms of screening for prostate cancer include harms from the PSA screening test and subsequent harms from diagnosis and treatment. Potential harms of screening include frequent false-positive results and psychological harms. One major trial in men screened every 2 to 4 years concluded that over 10 years, more than 15% of men experienced at least 1 false-positive test result.5 Harms of diagnostic procedures include complications of prostate biopsy, such as pain, hematospermia (blood in semen or ejaculate), and infection. Approximately 1% of prostate biopsies result in complications requiring hospitalization. The false-positive and complication rates from biopsy are higher in older men.3 Adequate evidence suggests that the harms of screening and diagnostic procedures are at least small.
PSA-based screening for prostate cancer leads to the diagnosis of prostate cancer in some men whose cancer would never have become symptomatic during their lifetime. Treatment of these men results in harms and provides them with no benefit. This is known as overdiagnosis, and follow-up of large randomized trials suggests that 20% to 50% of men diagnosed with prostate cancer through screening may be overdiagnosed.3 Overdiagnosis rates would be expected to increase with age and to be highest in men 70 years and older because older men have high risk of death from competing causes.
Harms of prostate cancer treatment include erectile dysfunction, urinary incontinence, and bothersome bowel symptoms. About 1 in 5 men who undergo radical prostatectomy develop long-term urinary incontinence requiring use of pads, and 2 in 3 men will experience long-term erectile dysfunction. More than half of men who receive radiation therapy experience long-term sexual erectile dysfunction and up to 1 in 6 men experience long-term bothersome bowel symptoms, including bowel urgency and fecal incontinence.3 Adequate evidence suggests that the harms of overdiagnosis and treatment are at least moderate.
Adequate evidence shows that the harms of screening in men older than 70 years are at least moderate and greater than in younger men because of increased risk of false-positive results, harms from diagnostic biopsy, and harms from treatment.
USPSTF ASSESSMENT
PSA-based screening for prostate cancer has both potential benefits and harms. The USPSTF does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks. The decision about whether to be screened for prostate cancer requires that each man incorporate his own values about the potential benefits and harms. The potential harms of screening, diagnostic procedures, and treatment occur soon after screening takes place. Although the potential benefits may occur any time after screening, they generally occur years after treatment because progression from asymptomatic, screen-detected cancer to symptomatic, metastasized cancer or death (if it occurs at all) may take years or decades to occur.
The USPSTF concludes with moderate certainty that the net benefit of PSA-based screening for prostate cancer in men aged 55 to 69 years is small for some men. How each man weighs specific benefits and harms will determine whether the overall net benefit is small.
The USPSTF concludes with moderate certainty that the potential benefits of PSA-based screening for prostate cancer in men 70 years and older do not outweigh the expected harms.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to adult men in the general U.S. population without symptoms or a previous diagnosis of prostate cancer. It also applies to men at increased risk of death from prostate cancer because of race/ethnicity or family history of prostate cancer. The sections below provide more information on how this recommendation applies to African American men and men with a family history of prostate cancer.
RISK ASSESSMENT
Older age, African American race, and family history of prostate cancer are the most important risk factors for the development of prostate cancer. Other factors with weaker associations and less evidence include diets high in fat and low in vegetable consumption. Cigarette smoking is associated with higher risk of prostate cancer mortality.
SCREENING
PSA-based screening is the usual method of screening and has been studied in several large trials. Although new screening methods are being developed (such as single- and adjusted-threshold testing and PSA velocity and doubling time), evidence is insufficient to support one method of PSA-based screening over another. Evidence is also insufficient that using a prebiopsy risk calculator, with or without measurement of free PSA levels, or using genetic or adjunctive imaging tests meaningfully changes the potential benefits and harms of screening. This is an important area of current research that has the potential to decrease the harms of PSA-based screening for prostate cancer. The use of digital rectal examination as a screening modality is not recommended because there is a lack of evidence on the benefits; digital rectal examination was either eliminated from or not included in the major screening trials.
PSA-based screening for prostate cancer has been studied in 3 very large RCTs, each with at least a decade of median follow-up: the U.S.-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, the European Randomized Study of Screening for Prostate Cancer (ERSPC), and the Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP). These trials used varying screening intervals (from 1-time screening to every 1 to 4 years) and PSA thresholds (2.5 to 10.0 ng/mL) for diagnostic biopsy.3
The PLCO trial may be viewed as a trial of organized vs. opportunistic screening for prostate cancer because of the substantial screening rate in the control group and the high screening rate among men in both the control and intervention groups prior to study enrollment.6 Men in the intervention group were screened more often than men in the control group, and more men in the intervention group were diagnosed with prostate cancer than in the control group. The trial found no difference between groups in death from prostate cancer after almost 15 years of follow-up: absolute risk, 4.8 per 1,000 person-years in the intervention group vs. 4.6 per 1,000 person-years in the control group; relative risk, 1.04 (95% confidence interval [CI], 0.87–1.24).7
In the ERSPC trial, the results suggest that, overall, the number needed to screen is 781 men aged 55 to 69 years at enrollment (95% CI, 490–1,929) to prevent 1 man from dying of prostate cancer after 13 years. The results varied across the individual ERSPC sites, and prostate cancer mortality was significantly reduced only at the sites in the Netherlands and Sweden. However, point estimates were in favor of screening at all sites except Switzerland. At the largest site (Finland), there was no significant benefit observed for prostate cancer mortality (rate ratio, 0.91 [95% CI, 0.75–1.10]), and in Sweden there was an absolute risk reduction of 0.72% (95% CI, 0.50%–0.94%), a 42% relative reduction.8–10
Four ERSPC trial sites reported data on the effect of PSA-based screening for prostate cancer on the development of metastatic cancer after 12 years of follow-up. The risk of developing metastatic prostate cancer was 30% lower among men randomized to screening than among men in the control group (absolute risk, 7.05 per 1,000 men in the screening group vs. 10.14 per 1,000 men in the control group [calculated from numbers in the study]). This translates to an absolute reduction in the long-term risk of metastatic prostate cancer of 3.1 cases per 1,000 men screened.11
The CAP trial was a cluster-randomized trial of a single invitation to PSA-based screening in the United Kingdom among 415,357 men. Overall, 34% of invited men received a valid PSA screening test. After a median follow-up of 10 years, there was no significant difference in prostate cancer mortality between the invited group and the control group (absolute risk, 0.30 per 1,000 person-years vs. 0.31 per 1,000 person-years, respectively).12
Based on clinical stage, tumor grade, and PSA level, prostate cancer is classified as low, medium, or high risk for clinical progression and prostate cancer death. Although treatment is thought to be most immediately beneficial for men with high- and medium-risk prostate cancer, the vast majority of cases of screen-detected cancer are low risk.
As with all screening tests, some men without prostate cancer will receive positive PSA test results (i.e., false-positive results). The false-positive rate for the PSA test depends on the PSA threshold used. Among 5 ERSPC sites that reported the false-positive rate, approximately 1 in 6 men screened at least once had 1 or more false-positive results, and of the positive results in the first round of screening, two-thirds were false positives. In Sweden, where a low PSA threshold (3.0 ng/mL) was used to determine a positive test result and men were screened every 2 years, more than 45% of men who participated in all screening rounds had a false-positive result over 10 years of screening.5 In the PLCO trial, more than two-thirds of men who underwent a prostate biopsy because of a positive PSA test result were found not to have prostate cancer.13 In addition to false-positive results, there are other harms associated with screening and subsequent diagnostic evaluation; biopsies may result in pain, fever, hematospermia, and hospitalization.
The 3 large RCTs on screening predominantly included men aged 55 to 69 years. There is inadequate evidence on starting screening at a younger age in the average-risk population or to obtain a baseline PSA level. Evidence in men 70 years and older does not support routine screening because of the lack of trial evidence of benefit, the low likelihood of benefit given the time to realize benefit, and the increased risk of harms from false-positive results, biopsies, overdiagnosis, and treatment. Although the evidence does not support routine screening in all men older than 70 years, the USPSTF recognizes the common use of PSA-based screening in practice today and understands that some older men will continue to request screening and some clinicians will continue to offer it. Men older than 70 years who request screening should be aware of the reduced likelihood of benefit from screening and the increased risk of false-positive test results and complications of diagnosis and treatment.
The USPSTF considered whether there are screening and follow-up approaches that increase the potential for benefit while reducing the potential for harms. Variation across sites in randomized trials of screening suggests there may be greater mortality benefit from screening every other year compared with longer intervals and from using lower PSA thresholds for diagnostic biopsy. Although these approaches may have increased the potential benefit reported in studies, they also resulted in substantially more harms—more false-positive results, more prostate biopsies, and more cases of overdiagnosis. This trade-off was also observed in a review of decision analysis models; screening protocols using lower PSA thresholds (< 4.0 ng/mL) for biopsy and more frequent screening intervals offered greater potential reductions in prostate cancer mortality but higher rates of overdiagnosis and other harms.14 The frequency of screening in the ERSPC sites ranged from every 2 to 7 years. No ERSPC trial site offered screening more often than every 2 years, and many sites screened every 4 years. The PSA threshold for biopsy in the ERSPC sites ranged from 2.5 to 4 ng/mL (except for 10 ng/mL in the earlier years at the Belgium site). In the Göteborg, Sweden, site, which reported the largest benefit, the frequency of screening was every 2 years, and the threshold for biopsy was 2.5 ng/mL (3.0 ng/mL in the first few years of the study).
TREATMENT
The potential benefit of screening for prostate cancer is because of treatment. Thus, it is important for men to consider both the potential benefits and harms of treatment (including active surveillance) as they consider whether to be screened. Men not able or willing to tolerate treatment should not be screened for prostate cancer. Because most cases of prostate cancer advance very slowly, if at all, the 10-year survival rate for screen-detected, localized prostate cancer is very high. In a recent major trial that enrolled more than 1,500 men randomized to receive either active treatment or active surveillance, the 10-year survival rate in all groups was 99%.15 The good prognosis for early-stage prostate cancer makes it difficult to study the effectiveness of treatment.
Multiple treatment options exist for prostate cancer, and new ones are being developed. In current practice, the 3 most common treatment options for men with screen-detected, localized prostate cancer are surgical removal of the prostate gland (radical prostatectomy), radiation therapy (external-beam radiation therapy, proton beam therapy, or brachytherapy), and active surveillance. The USPSTF considered available evidence on treatment when evaluating the effectiveness of screening and found that current evidence suggests that treatment of early-stage, screen-detected prostate cancer with radical prostatectomy or radiation therapy likely reduces risk of clinical progression and metastatic disease and may reduce prostate cancer mortality. More details about the effectiveness and adverse effects of active treatment are provided in the Discussion section.
Active surveillance is a treatment approach that seeks to limit the harms of treatment by allowing men with apparent low-risk prostate cancer to forego surgery or radiation in favor of ongoing monitoring of their cancer. Although protocols vary, active surveillance usually includes regular, repeated PSA testing and often repeated digital rectal examination and prostate biopsy, with potential for exposure to repeated harms from biopsies. Men whose cancer is found to be changing are offered definitive treatment with surgery or radiation therapy. Other treatment monitoring strategies for men with low-risk cancer exist (for example, watchful waiting) and also vary in protocol. Active surveillance has become a more common treatment choice in the United States during the past several years. In a study assessing community-based urology practice in the United States between 2010 and 2013, about half of men with low-risk prostate cancer were treated with radical prostatectomy. The active surveillance rate, however, increased from about 10% in 2005–2009 to 40.4% in 2010–2013 among men with low-risk prostate cancer.16
Active treatment of prostate cancer can result in major adverse effects. About 3 in 1,000 men die during or soon after radical prostatectomy, and about 50 in 1,000 men have serious surgical complications requiring intervention. About 1 in 5 men who undergo radical prostatectomy develop long-term urinary incontinence requiring regular use of pads, and about 2 in 3 men experience long-term erectile dysfunction. More than half of men who receive radiation therapy experience long-term erectile dysfunction, and up to 1 in 6 men experience long-term bothersome bowel symptoms, including bowel urgency and fecal incontinence.3
SCREENING FOR PROSTATE CANCER IN AFRICAN AMERICAN MEN
Burden. In the United States, African American men are more likely to develop prostate cancer than white men (203.5 vs. 121.9 cases per 100,000 men). African American men are also more than twice as likely as white men to die of prostate cancer (44.1 vs. 19.1 deaths per 100,000 men).1 The higher death rate is attributable in part to an earlier age at cancer onset, more advanced cancer stage at diagnosis, and higher rates of more aggressive cancer (i.e., higher tumor grade). These differences in death from prostate cancer may also reflect that African American men have lower rates of receiving high-quality care.
Available Evidence. The USPSTF searched for evidence about the potential benefits and harms of PSA-based screening for prostate cancer in African American men.
Potential Benefits. The PLCO trial enrolled 4% African American men, which is not enough to determine whether the overall trial results differed for African American men.17 The ERSPC trial did not record or report any race-specific subgroup information. The low proportion of persons of African descent in European countries during the study period makes it likely that these groups were not well represented.
Potential Harms. An analysis from the PLCO trial found that African American men were significantly more likely to have major infections after prostate biopsy than white men (odds ratio, 7.1 [95% CI, 2.7–18.0]).13 Evidence is insufficient to compare the risk of false-positive results, potential for overdiagnosis, and magnitude of harms from prostate cancer treatment in African American vs. other men.
Advising African American Men. Based on the available evidence, the USPSTF is not able to make a separate, specific recommendation on PSA-based screening for prostate cancer in African American men. Although it is possible that screening may offer greater benefits for African American men compared with the general population, currently no direct evidence demonstrates whether this is true. Screening, and subsequent diagnosis and treatment, has the potential to increase exposure to potential harms. Decision analysis models suggest that given the higher rates of aggressive prostate cancer in African American men, PSA-based screening may provide greater benefit to African American men than the general population. These models also suggest a potential mortality benefit for African American men when beginning screening before age 55 years. The USPSTF believes that a reasonable approach for clinicians is to inform African American men about their increased risk of developing and dying of prostate cancer as well as the potential benefits and harms of screening so they can make an informed, personal decision about whether to be screened. Although the USPSTF found inadequate evidence about how benefits may differ for African American men, it recognizes the epidemiologic data showing that African American men may develop prostate cancer at younger ages than average-risk men and understands that some African American men and their clinicians will continue to screen at younger ages. The USPSTF does not recommend screening for prostate cancer in men, including African American men, older than 70 years.
The USPSTF strongly encourages research on screening for and treatment of prostate cancer in African American men. It is important to consider both the potential additional benefits and harms to fully understand the value of screening. Studies are needed to confirm that African American men who undergo screening receive similar or greater reductions in prostate cancer mortality compared with men in the general population, as well as to explore the optimal screening frequency and whether beginning screening before age 55 years provides additional benefits in African American men. Studies are also needed to better understand strategies to mitigate harms and maximize benefits of screening, diagnostic follow-up, and treatment (including active surveillance) in African American men. It is also important that research and quality improvement activities continue to work to eliminate disparities in access to high-quality care for men with prostate cancer.
SCREENING FOR PROSTATE CANCER IN MEN WITH A FAMILY HISTORY
Burden. The introduction of PSA-based screening for prostate cancer has substantially altered the epidemiologic data for prostate cancer, greatly increasing the number of men with a diagnosis of prostate cancer and thus also the number of men with a father, brother, or son with a history of prostate cancer.
Available Evidence. It is generally accepted that men with a family history of prostate cancer are more likely to develop prostate cancer. A study of twins in Scandinavia estimated that genetic factors may account for up to 42% of prostate cancer risk.18 An analysis from the Finnish site of the ERSPC trial concluded that men with at least 1 first-degree relative with prostate cancer were 30% more likely to be diagnosed with prostate cancer than men without a family history.19 Men with 3 first-degree relatives with prostate cancer or 2 close relatives on the same side of the family with prostate cancer diagnosed before age 55 years may have an inheritable form of prostate cancer associated with genetic changes passed down from one generation to the next. This type of prostate cancer is thought to account for less than 10% of all prostate cancer cases.20
The USPSTF searched for evidence about the potential benefits and harms of PSA-based screening for prostate cancer in men with a family history of prostate cancer.
Potential Benefits. Of the 7% of men in the PLCO trial who reported a family history of prostate cancer on a baseline questionnaire, prostate cancer mortality was lower among white men in the intervention group than in the control group (hazard ratio, 0.49 [95% CI, 0.22–1.10]; P = .08),21 but the difference was not significant and the CI was wide.
Potential Harms. No studies have assessed the risk of harms related to screening for, diagnosis of, or treatment of prostate cancer based on family history of prostate cancer.
Advising Men with a Family History of Prostate Cancer. Based on the available evidence, the USPSTF is not able to make a separate, specific recommendation on PSA-based screening for prostate cancer in men with a family history of prostate cancer. Although it is possible that screening may offer additional potential benefits for these men compared with the general population, screening also has the potential to increase exposure to potential harms, especially among men with relatives whose cancer was overdiagnosed. Men who have a first-degree relative who had advanced prostate cancer at diagnosis, developed metastatic prostate cancer, or died of prostate cancer are probably the most likely to benefit from screening. The USPSTF believes that a reasonable approach for clinicians is to inform men with a family history of prostate cancer, particularly those with multiple first-degree relatives with prostate cancer, about their increased risk of developing cancer as well as the potential earlier age at disease onset. This discussion should include the potential benefits and harms of screening for prostate cancer so these men have the opportunity to make an informed, personal decision about whether to be screened. Although the USPSTF found inadequate evidence about how benefits may differ for men with a family history of prostate cancer, it recognizes the epidemiologic data showing that these men are at a greater than average risk and understands that some men and their clinicians will continue to screen at younger ages in men with a family history. The USPSTF does not recommend screening for prostate cancer in men, including men with a family history of prostate cancer, older than 70 years.
Epidemiologic studies examining outcomes in men with relatives who died of prostate cancer vs. men with relatives diagnosed with prostate cancer who died of other causes may help provide better guidance. Studies are needed that explore the optimal screening frequency and whether beginning screening before age 55 years provides additional benefits for men with a family history of prostate cancer. Additional research is also needed to help identify men with an inheritable form of prostate cancer and to understand how the potential benefits and harms of screening, including screening intervals and starting ages, may differ in these men compared with the general population.
RESEARCH NEEDS AND GAPS
There are many areas in need of research to improve screening for and treatment of prostate cancer, including:
Comparing different screening strategies, including different screening intervals, to fully understand the effects on benefits and harms
Developing, validating, and providing longer term follow-up of screening and diagnostic techniques, including risk stratification tools, use of baseline PSA level as a risk factor, and use of non–PSA-based adjunctive tests that can distinguish nonprogressive and slowly progressive cancer from cancer that is likely to become symptomatic and affect quality or length of life, to reduce overdiagnosis and overtreatment
Screening for and treatment of prostate cancer in African American men, including understanding the potential benefits and harms of different starting ages and screening intervals and the use of active surveillance; given the large disparities in prostate cancer mortality in African American men, this should be a national priority
How to better inform men with a family history of prostate cancer about the benefits and harms of PSA-based screening for prostate cancer, including the potential differences in outcomes between men with relatives who died of prostate cancer and men with relatives diagnosed with prostate cancer who died of other causes
How to refine active prostate cancer treatments to minimize harms
How to better understand patient values about the known benefits and harms of screening for and treatment of prostate cancer; how these values influence men's assessment of the overall benefit vs. harm; how to best implement informed decision-making programs that incorporate the values and preferences of men and their families about screening; how to adapt the informed decision-making process to a range of diverse patient populations as screening, diagnosis, and treatment strategies evolve; and the effects of informed decision making on health outcomes and patient experience
