
Am Fam Physician. 2018;98(12):751-753
Author disclosure: No relevant financial affiliations.
Clinical Question
In patients with nonvalvular atrial fibrillation (NVAF), what is the best way to determine if the benefits of stroke reduction exceed the harms of bleeding from anticoagulants?
Evidence Summary
In patients with NVAF, anticoagulants decrease the risk of stroke by approximately 60%.1 However, they also significantly increase the risk of a major bleeding event or intracranial hemorrhage. Thus, the decision to use anticoagulants in patients with NVAF should be guided by an assessment of potential benefit (reduction in stroke risk) and potential harm (increase in bleeding risk).
A number of clinical decision rules have been proposed to predict the risk of stroke in patients with NVAF. The 2018 American College of Chest Physicians guidelines for antithrombotic therapy in the management of NVAF recommend using the CHA2DS2-VASc score2 (Table 13–6). Men with a score of 0 and women with a score of 1 are considered low risk and do not require antithrombotic therapy with anticoagulants or aspirin. For all other patients, treatment with a direct oral anticoagulant is recommended. Patients at high risk of bleeding based on the validated HAS-BLED score (Table 13–6) should be monitored closely during treatment for signs of bleeding, and modifiable risk factors for bleeding should be addressed.2
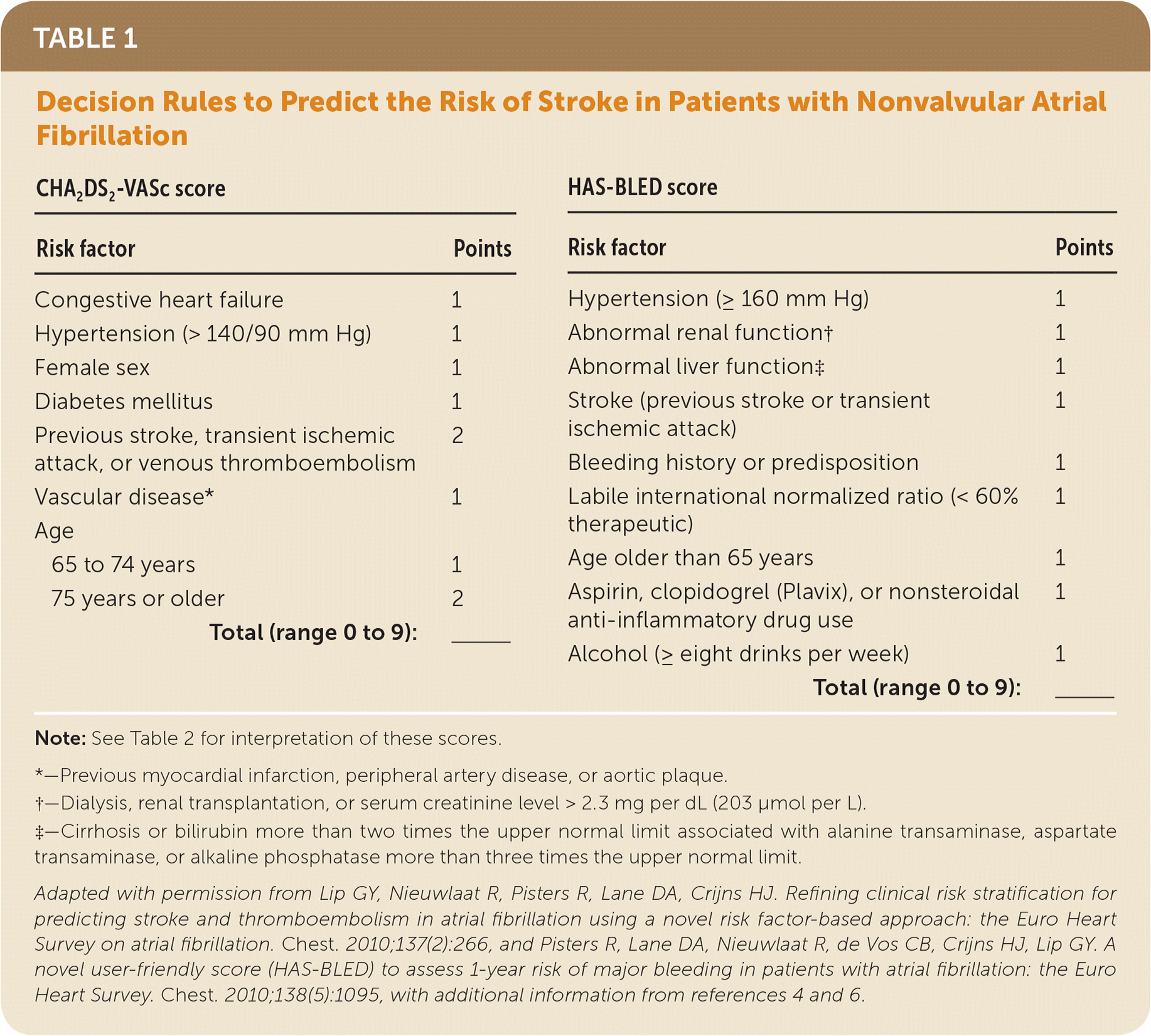
| CHA2DS2-VASc score | HAS-BLED score | |||
|---|---|---|---|---|
| Risk factor | Points | Risk factor | Points | |
| Congestive heart failure | 1 | Hypertension (≥ 160 mm Hg) | 1 | |
| Hypertension (> 140/90 mm Hg) | 1 | Abnormal renal function† | 1 | |
| Female sex | 1 | Abnormal liver function‡ | 1 | |
| Diabetes mellitus | 1 | Stroke (previous stroke or transient ischemic attack) | 1 | |
| Previous stroke, transient ischemic attack, or venous thromboembolism | 2 | Bleeding history or predisposition | 1 | |
| Vascular disease* | 1 | Labile international normalized ratio (< 60% therapeutic) | 1 | |
| Age | Age older than 65 years | 1 | ||
| 65 to 74 years | 1 | Aspirin, clopidogrel (Plavix), or nonsteroidal anti-inflammatory drug use | 1 | |
| 75 years or older | 2 | Alcohol (≥ eight drinks per week) | 1 | |
| Total (range 0 to 9): | ______ | Total (range 0 to 9): | ______ | |
The 2016 guidelines from the European Society of Cardiology also recommend using the CHA2DS2-VASc score to assess the risk of stroke.7 They are similar to the American College of Chest Physicians guidelines, recommending no antithrombotic therapy for men with a score of 0 and for women with a score of 1. They differ in recommending that oral anticoagulation be considered, but is not mandatory, for men with a score of 1 and women with a score of 2. Anticoagulation should be initiated for patients with higher scores, however.7
Guidelines should be applied with the individual patient in mind, considering factors such as comorbidities, history of falls, previous episodes of bleeding and intracranial hemorrhage, and patient values. An important factor is the overall risk of stroke in patients with NVAF who do not receive anticoagulation. A systematic review identified eight studies of such patients, with the risk of ischemic stroke ranging from 2.0 to 4.5 per 100 person-years. Both the median and weighted mean were 3.0 strokes per 100 person-years.6 Thus, a 60% reduction in the risk of stroke with anticoagulation would reduce the number of strokes to approximately 1.2 per 1080 person-years (number needed to treat to prevent one stroke in one year = 56). A cost-utility analysis concluded that the optimal threshold for recommending anticoagulation using warfarin (Coumadin) in a patient with NVAF is an annual stroke risk of 1.7%, with patients below that threshold receiving aspirin only.8
A meta-analysis of four studies including patients with NVAF who did not receive anticoagulation showed a stroke risk of 0.2, 0.7, and 1.5 strokes per 100 person-years for unselected patients with a CHA2DS2-VASc score of 0, 1, or 2, respectively.6 A large prospective cohort study with 182,678 patients found generally similar stroke risks in patients not receiving an anticoagulant and also calculated HAS-BLED scores in the same cohort for patients receiving an oral anticoagulant.9 Data from the cohort study regarding the risk of bleeding and stroke and the potential benefits and harms of anticoagulation in patients with NVAF based on CHA2DS2-VASc and HAS-BLED scores are shown in Table 2.1,9
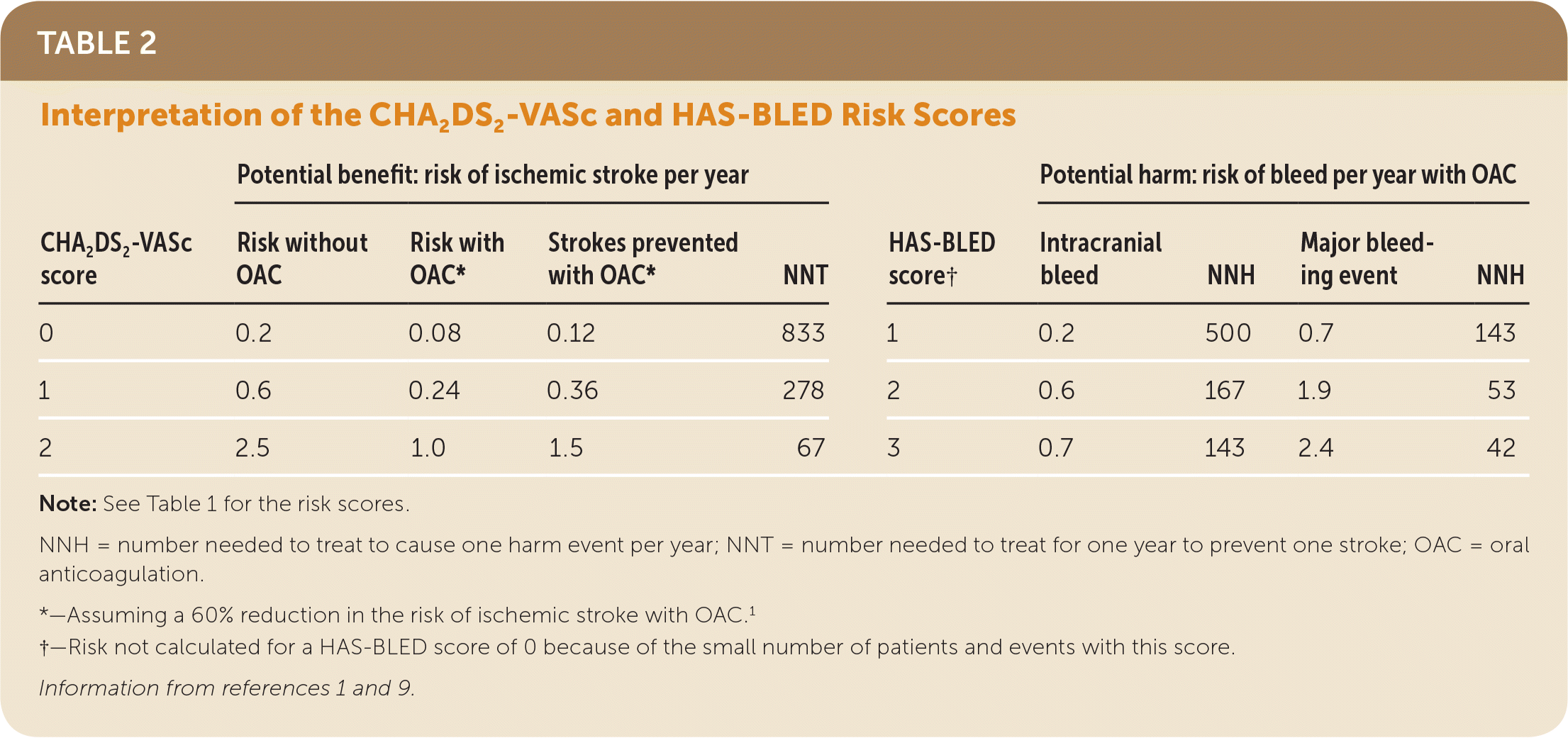
| Potential benefit: risk of ischemic stroke per year | Potential harm: risk of bleed per year with OAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CHA2DS2-VASc score | Risk without OAC | Risk with OAC* | Strokes prevented with OAC* | NNT | HAS-BLED score† | Intracranial bleed | NNH | Major bleeding event | NNH |
| 0 | 0.2 | 0.08 | 0.12 | 833 | 1 | 0.2 | 500 | 0.7 | 143 |
| 1 | 0.6 | 0.24 | 0.36 | 278 | 2 | 0.6 | 167 | 1.9 | 53 |
| 2 | 2.5 | 1.0 | 1.5 | 67 | 3 | 0.7 | 143 | 2.4 | 42 |
The likelihood of preventing a stroke is less than the increased risk of an intracranial hemorrhage for patients with a CHA2DS2-VASc score of 0 or 1 and an average bleeding risk. For patients with a CHA2DS2-VASc score of 2, it is estimated that 1.5 strokes are prevented with anticoagulation, whereas 0.6 intracranial bleeds and 2.3 major bleeding events are caused by anticoagulation in a person at average bleeding risk.1,9 The risk assessment is different for patients at lower or higher bleeding risk.
Applying the Evidence
A 69-year-old man with a history of hypertension but no other risk factors for stroke is diagnosed with NVAF. His CHA2DS2-VASc score is 2 (one point each for hypertension and age). He takes a nonsteroidal anti-inflammatory drug daily for osteoarthritis; therefore, his HAS-BLED score is 3 (one point each for hypertension, age, and nonsteroidal anti-inflammatory drug use). Anticoagulation will reduce his risk of stroke by 1.5% per year (number needed to treat per year = 67), whereas with anticoagulation he would have a 0.7% risk of an intracranial bleed (number needed to harm = 143) and a 2.4% risk of a major bleeding event (number needed to harm = 42). The patient has advanced Parkinson disease and mild cognitive impairment. Based on the patient’s and his caregiver’s evaluation of potential benefits and harms, he decides to forgo anticoagulation.
