Am Fam Physician. 2019;99(2):109-116
Related letter: Substance Use Disorders: Considerations in Maternity and Neonatal Care
Author disclosure: No relevant financial affiliations.
More than 750,000 persons in the United States inject opioids, methamphetamine, cocaine, or ketamine, and that number is increasing because of the current opioid epidemic. Persons who inject drugs (PWID) are at higher risk of infectious and noninfectious skin, pulmonary, cardiac, neurologic, and other causes of morbidity and mortality. Nonjudgmental inquiries about current drug use can uncover information about readiness for addiction treatment and identify modifiable risk factors for complications of injection drug use. All PWID should be screened for human immunodeficiency virus infection, latent tuberculosis, and hepatitis B and C, and receive vaccinations for hepatitis A and B, tetanus, and pneumonia if indicated. Pre-exposure prophylaxis for human immunodeficiency virus infection should also be offered. Naloxone should be prescribed to those at risk of opioid overdose. Skin and soft tissue infections are the most common medical complication in PWID and the top reason for hospitalization in these patients. Signs of systemic infection require hospitalization, blood cultures, and a comprehensive history and physical examination to determine the source of infection. PWID have a higher incidence of community-acquired pneumonia and are at risk of other pulmonary complications, including opioid-associated pulmonary edema, asthma, and foreign body granulomatosis. Infectious endocarditis is the most common cardiac complication associated with injection drug use and more often involves the right-sided heart valves, which may not present with heart murmurs or peripheral signs and symptoms, in PWID. Injections increase the risk of osteomyelitis, as well as subdural and epidural abscesses.
In the United States, more than 6.5 million persons have injected drugs, and more than 750,000 currently use injection drugs.1 The number of persons who inject drugs (PWID) has grown sharply in recent years with the rise of the opioid epidemic.2 Opioids and methamphetamines are the most commonly injected drugs. Cocaine and ketamine are also injected but less often.3
| Clinical recommendation | Evidence | References |
|---|---|---|
| Buprenorphine or methadone should be offered to PWID for opioid detoxification and medication-assisted treatment. : | A | 18, 19, 21 |
| All PWID should be screened for | ||
| Hepatitis B and C | B | 22–24 |
| Human immunodeficiency virus infection | A | 25 |
| Latent tuberculosis | C | 26, 27 |
| All PWID should receive hepatitis A and hepatitis B vaccinations, and be up to date on tetanus vaccinations. | C | 30, 32 |
| All eligible PWID should be offered pre-exposure prophylaxis for human immunodeficiency virus infection. | B | 34, 42 |
| Naloxone should be prescribed to PWID at high risk of opioid overdose. | C | 35 |
| Safer injecting practices should be discussed with all PWID. | C | 30, 43 |
PWID have higher morbidity and mortality from numerous causes, including infections (predominantly human immunodeficiency virus [HIV] infection; hepatitis; endocarditis; and pulmonary, bone, and skin infections), psychiatric disorders (e.g., major depression, generalized anxiety disorder, posttraumatic stress disorder, personality disorders), violence, and accidents.4–6 PWID have higher mortality than the general population, with a crude mortality rate of 2.64 per 100 years of active injecting.7,8
Initial Evaluation
PWID are less likely to receive primary care than the general population.9 However, family physicians can screen patients for illicit drug use, as well as provide brief intervention and referral to treatment. Although the U.S. Preventive Services Task Force found in 2008 that the evidence was insufficient to merit a recommendation on illicit drug screening, it is currently reevaluating the evidence.10
Physicians can screen for injection drug use through routine, open-ended questions in the social history or through validated screening questionnaires such as the Drug Abuse Screening Test (available at https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/228) or the modified Alcohol, Smoking and Substance Involvement Screening Test (available at https://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf).11,12 Nonjudgmental inquiries about current drug use can uncover information about readiness for addiction treatment and identify modifiable risk factors for complications of injection drug use. Some PWID may be ready to seek additional medical services for opioid use disorder, whereas others may be precontemplative.13 Using motivational interviewing techniques (e.g., collaboration, empathy) can help foster motivation for change.14
Physical signs of active injection drug use include recent injection sites, bruising, and patches of hyperpigmentation. These are most common in the antecubital fossa but can also be found elsewhere (e.g., upper arms, hands, neck, groin, fingers, toes) if venous sclerosis occurs at more easily accessible sites.15
Family physicians have multiple office-based options for patients with opioid use disorder who want treatment, including sublingual buprenorphine, oral naltrexone (Revia), and depot naltrexone (Vivitrol).16 Prescribing of buprenorphine was outlined in a previous issue of American Family Physician.17 Maintenance therapy with buprenorphine or methadone significantly reduces opioid use, increases treatment retention, lowers overall mortality, and improves physical and mental health.18–20
Patients with opioid use disorder who request medically supervised withdrawal, or detoxification, can be treated with antiemetics (prochlorperazine, ondansetron [Zofran]), antidiarrheals (loperamide [Imodium]), and sedatives (trazodone, doxepin) to alleviate the specific symptoms of opioid withdrawal. Alpha-2 agonists such as lofexidine (Lucemyra) and clonidine (off-label use) can also be used. Buprenorphine tapers are significantly more effective than nonopioid therapy in decreasing the discomfort of opioid withdrawal and keeping patients in treatment.19,21
Preventive Care Recommendations
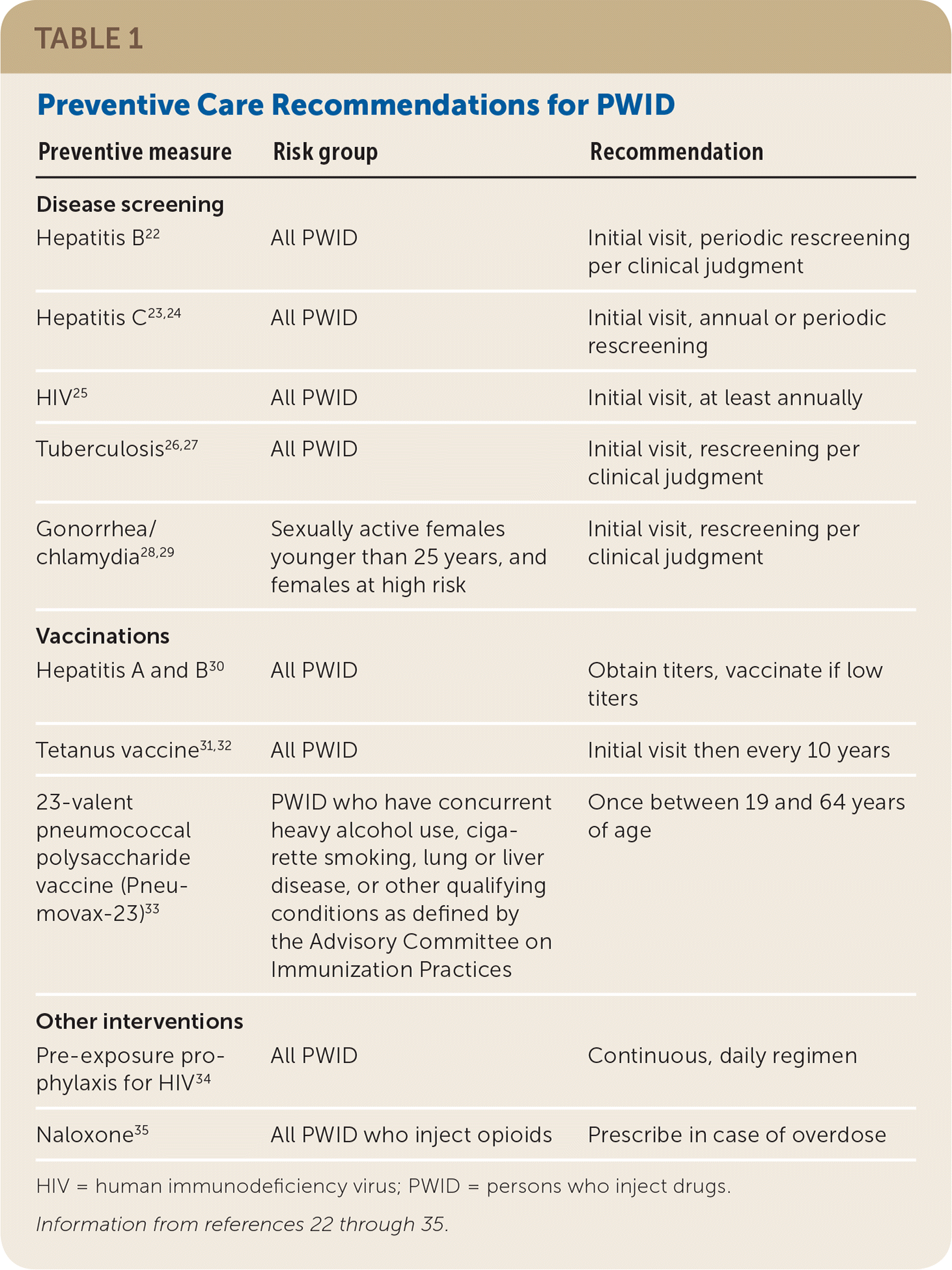
| Preventive measure | Risk group | Recommendation |
|---|---|---|
| Disease screening | ||
| Hepatitis B22 | All PWID | Initial visit, periodic rescreening per clinical judgment |
| Hepatitis C23,24 | All PWID | Initial visit, annual or periodic rescreening |
| HIV25 | All PWID | Initial visit, at least annually |
| Tuberculosis26,27 | All PWID | Initial visit, rescreening per clinical judgment |
| Gonorrhea/chlamydia28,29 | Sexually active females younger than 25 years, and females at high risk | Initial visit, rescreening per clinical judgment |
| Vaccinations | ||
| Hepatitis A and B30 | All PWID | Obtain titers, vaccinate if low titers |
| Tetanus vaccine31,32 | All PWID | Initial visit then every 10 years |
| 23-valent pneumococcal polysaccharide vaccine (Pneumovax-23)33 | PWID who have concurrent heavy alcohol use, cigarette smoking, lung or liverdisease, or other qualifying conditions as defined by the Advisory Committee on Immunization Practices | Once between 19 and 64 years of age |
| Other interventions | ||
| Pre-exposure prophylaxis for HIV34 | All PWID | Continuous, daily regimen |
| Naloxone35 | All PWID who inject opioids | Prescribe in case of overdose |
INFECTIOUS DISEASE SCREENING AND TREATMENT
The U.S. Preventive Services Task Force recommends screening for HIV infection and hepatitis B and C in PWID.22,23,25 At least annual screening for HIV and hepatitis C is suggested.24,25 Latent tuberculosis testing is also recommended for PWID.26,27 Screening and behavioral counseling for gonorrhea and chlamydia are recommended for sexually active females younger than 25 years and for females engaged in high-risk sexual behaviors.28,29
PWID who test positive for HIV infection or hepatitis C should receive antiviral treatment. Active injection drug use is not a contraindication for HIV or hepatitis C treatment because successful treatment greatly reduces the risk of viral transmission, limiting spread in the community at large.36–38
VACCINATIONS
All PWID should receive hepatitis A and B vaccinations if there is no evidence of immunity from vaccine titers30 and be up to date on tetanus vaccinations.31,32 Although PWID have an estimated 10-fold greater risk of community-acquired pneumonia,39 the Centers for Disease Control and Prevention does not consider active injection drug use alone as an indication for early pneumonia vaccine.33,39 However, a one-time 23-valent pneumococcal polysaccharide vaccine (Pneumovax-23) is indicated between 19 and 64 years of age in those with certain concurrent conditions, such as heavy alcohol use, cigarette smoking, or lung or liver disease.33
PRE-EXPOSURE PROPHYLAXIS FOR HIV
Of new HIV infections in the United States, 8% to 12% occur in PWID, as a result of injecting or injecting in conjunction with high-risk sexual practices.40,41 Pre-exposure prophylaxis with the tenofovir/emtricitabine combination (Truvada; preferred drug) dramatically reduces the risk of HIV transmission in PWID.42 The Centers for Disease Control and Prevention recommends daily, continuous pre-exposure prophylaxis for adults who have injected drugs within the previous six months, and have also shared injection or drug preparation equipment in the previous six months or engaged in high-risk sexual practices.34
Patients are eligible for pre-exposure prophylaxis if they have negative HIV test results, no signs of HIV infection within the previous four weeks, normal creatinine clearance, and laboratory evidence of immunity to hepatitis B virus or absence of infection without immunity (in which case they should be vaccinated against hepatitis B).34
Clinicians should monitor for symptoms suggestive of acute HIV infection (i.e., fever, fatigue, myalgias, skin rash, headache, pharyngitis, cervical adenopathy). For patients on a pre-exposure prophylaxis regimen, repeat HIV and pregnancy testing (if applicable) is indicated every three months, and creatinine clearance testing and sexually transmitted infection screening are recommended every six months.34 Centers for Disease Control and Prevention guidelines for prescribing pre-exposure prophylaxis are available at https://www.cdc.gov/hiv/risk/prep/index.html.
NALOXONE FOR OPIOID OVERDOSE
Naloxone is a synthetic opioid antagonist with high opioid receptor affinity that reverses potentially fatal respiratory depression through competitive inhibition. It is available in several forms and strengths, including an intramuscular form, a preassembled intranasal kit (Narcan), and an autoinjector (Evzio). Naloxone can lead to unpleasant but nonfatal acute withdrawal symptoms in chronic opioid users, including agitation, anxiety, lacrimation, rhinorrhea, diarrhea, nausea, and vomiting. All patients should be instructed to seek medical attention after administration of naloxone given its shorter half-life compared with most opioids. Close observation is needed because readministration may be required. More information on prescribing naloxone is available at http://prescribetoprevent.org.
The American Academy of Family Physicians recommends providing access to appropriate overdose antidotes, such as naloxone prescriptions, for individuals at high risk of opioid overdose.35 Risk factors for overdose include recent abstinence from opioid use, hospitalization, or incarceration; history of overdose; chronic hepatic or pulmonary conditions; or mental health conditions.
SAFER INJECTING PRACTICES
Needle-syringe exchange programs reduce rates of HIV, hepatitis C, and high-risk injecting behavior without increasing drug use, number of PWID, or needles discarded in an unsafe manner.43–45 The American Academy of Family Physicians supports the use of these exchange programs.46 Program locations can be found at https://www.nasen.org/map/.
Alternatively, physicians in the District of Columbia and all states except Delaware and Kansas are allowed to prescribe or dispense syringes to PWID. Information regarding the laws in each state is available at http://www.temple.edu/lawschool/aidspolicy/50statesataglance.htm.
Physicians should counsel PWID about safer injection practices such as never reuse or share syringes; only use new or sterile syringes from reliable sources; use clean water, a new or disinfected container (cooker), and a new filter (cotton) to prepare drugs; use an alcohol swab to clean the injection site; and dispose of syringes after a single use.30,43
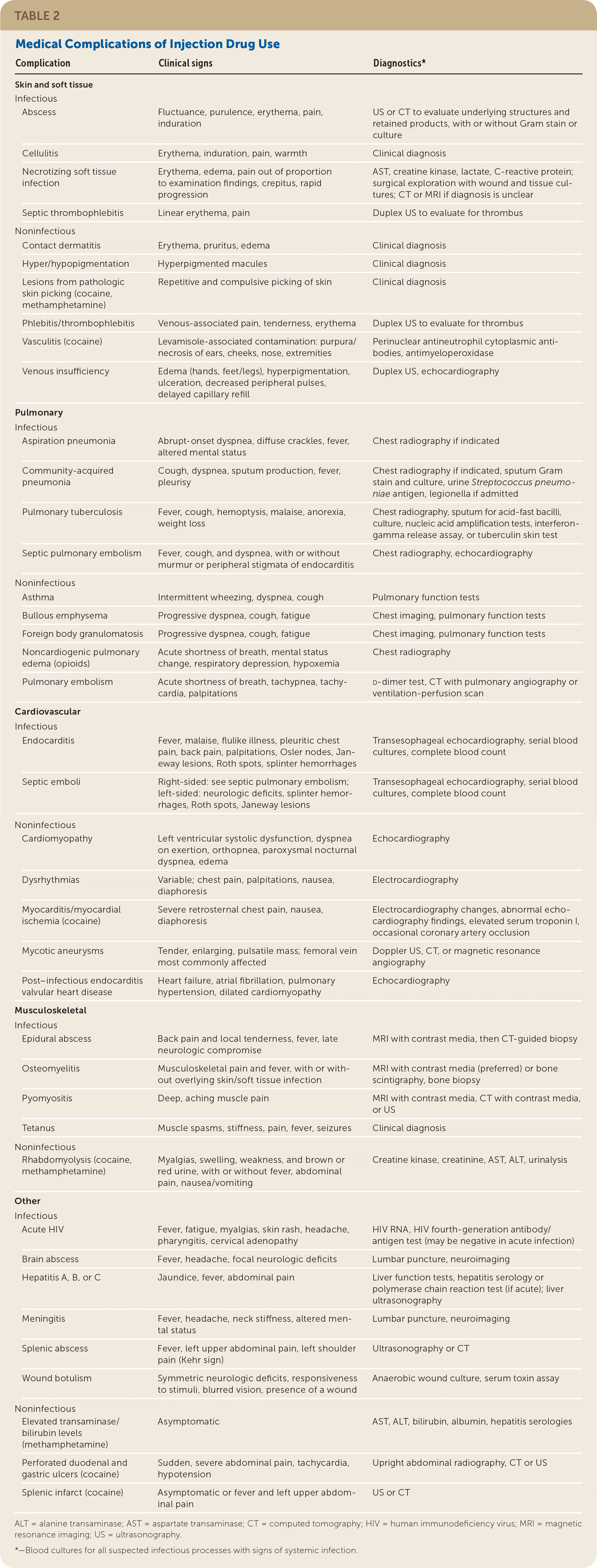
Medical Complications of Injection Drug Use
Injection drug use can result in harmful infectious and noninfectious effects to almost every organ system (Table 2). All PWID exhibiting signs of systemic infection, including fever, tachycardia, tachypnea, hypotension, and leukocytosis, should be referred to an emergency department or admitted to an acute care hospital for blood cultures and a full history and physical examination to determine the source of potential infections.
| Complication | Clinical signs | Diagnostics* |
|---|---|---|
| Skin and soft tissue | ||
| Infectious | ||
| Abscess | Fluctuance, purulence, erythema, pain, induration | US or CT to evaluate underlying structures and retained products, with or without Gram stain or culture |
| Cellulitis | Erythema, induration, pain, warmth | Clinical diagnosis |
| Necrotizing soft tissue infection | Erythema, edema, pain out of proportion to examination findings, crepitus, rapid progression | AST, creatine kinase, lactate, C-reactive protein; surgical exploration with wound and tissue cultures; CT or MRI if diagnosis is unclear |
| Septic thrombophlebitis | Linear erythema, pain | Duplex US to evaluate for thrombus |
| Noninfectious | ||
| Contact dermatitis | Erythema, pruritus, edema | Clinical diagnosis |
| Hyper/hypopigmentation | Hyperpigmented macules | Clinical diagnosis |
| Lesions from pathologic skin picking (cocaine, methamphetamine) | Repetitive and compulsive picking of skin | Clinical diagnosis |
| Phlebitis/thrombophlebitis | Venous-associated pain, tenderness, erythema | Duplex US to evaluate for thrombus |
| Vasculitis (cocaine) | Levamisole-associated contamination: purpura/necrosis of ears, cheeks, nose, extremities | Perinuclear antineutrophil cytoplasmic antibodies, antimyeloperoxidase |
| Venous insufficiency | Edema (hands, feet/legs), hyperpigmentation, ulceration, decreased peripheral pulses, delayed capillary refill | Duplex US, echocardiography |
| Pulmonary | ||
| Infectious | ||
| Aspiration pneumonia | Abrupt-onset dyspnea, diffuse crackles, fever, altered mental status | Chest radiography if indicated |
| Community-acquired pneumonia | Cough, dyspnea, sputum production, fever, pleurisy | Chest radiography if indicated, sputum Gram stain and culture, urine Streptococcus pneumoniae antigen, legionella if admitted |
| Pulmonary tuberculosis | Fever, cough, hemoptysis, malaise, anorexia, weight loss | Chest radiography, sputum for acid-fast bacilli, culture, nucleic acid amplification tests, interferon-gamma release assay, or tuberculin skin test |
| Septic pulmonary embolism | Fever, cough, and dyspnea, with or without murmur or peripheral stigmata of endocarditis | Chest radiography, echocardiography |
| Noninfectious | ||
| Asthma | Intermittent wheezing, dyspnea, cough | Pulmonary function tests |
| Bullous emphysema | Progressive dyspnea, cough, fatigue | Chest imaging, pulmonary function tests |
| Foreign body granulomatosis | Progressive dyspnea, cough, fatigue | Chest imaging, pulmonary function tests |
| Noncardiogenic pulmonary edema (opioids) | Acute shortness of breath, mental status change, respiratory depression, hypoxemia | Chest radiography |
| Pulmonary embolism | Acute shortness of breath, tachypnea, tachycardia, palpitations | d-dimer test, CT with pulmonary angiography or ventilation-perfusion scan |
| Cardiovascular | ||
| Infectious | ||
| Endocarditis | Fever, malaise, flulike illness, pleuritic chest pain, back pain, palpitations, Osler nodes, Janeway lesions, Roth spots, splinter hemorrhages | Transesophageal echocardiography, serial blood cultures, complete blood count |
| Septic emboli | Right-sided: see septic pulmonary embolism; left-sided: neurologic deficits, splinter hemorrhages, Roth spots, Janeway lesions | Transesophageal echocardiography, serial blood cultures, complete blood count |
| Noninfectious | ||
| Cardiomyopathy | Left ventricular systolic dysfunction, dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, edema | Echocardiography |
| Dysrhythmias | Variable; chest pain, palpitations, nausea, diaphoresis | Electrocardiography |
| Myocarditis/myocardial ischemia (cocaine) | Severe retrosternal chest pain, nausea, diaphoresis | Electrocardiography changes, abnormal echocardiography findings, elevated serum troponin I, occasional coronary artery occlusion |
| Mycotic aneurysms | Tender, enlarging, pulsatile mass; femoral vein most commonly affected | Doppler US, CT, or magnetic resonance angiography |
| Post–infectious endocarditis valvular heart disease | Heart failure, atrial fibrillation, pulmonary hypertension, dilated cardiomyopathy | Echocardiography |
| Musculoskeletal | ||
| Infectious | ||
| Epidural abscess | Back pain and local tenderness, fever, late neurologic compromise | MRI with contrast media, then CT-guided biopsy |
| Osteomyelitis | Musculoskeletal pain and fever, with or without overlying skin/soft tissue infection | MRI with contrast media (preferred) or bone scintigraphy, bone biopsy |
| Pyomyositis | Deep, aching muscle pain | MRI with contrast media, CT with contrast media, or US |
| Tetanus | Muscle spasms, stiffness, pain, fever, seizures | Clinical diagnosis |
| Noninfectious | ||
| Rhabdomyolysis (cocaine, methamphetamine) | Myalgias, swelling, weakness, and brown or red urine, with or without fever, abdominal pain, nausea/vomiting | Creatine kinase, creatinine, AST, ALT, urinalysis |
| Other | ||
| Infectious | ||
| Acute HIV | Fever, fatigue, myalgias, skin rash, headache, pharyngitis, cervical adenopathy | HIV RNA, HIV fourth-generation antibody/antigen test (may be negative in acute infection) |
| Brain abscess | Fever, headache, focal neurologic deficits | Lumbar puncture, neuroimaging |
| Hepatitis A, B, or C | Jaundice, fever, abdominal pain | Liver function tests, hepatitis serology or polymerase chain reaction test (if acute); liver ultrasonography |
| Meningitis | Fever, headache, neck stiffness, altered mental status | Lumbar puncture, neuroimaging |
| Splenic abscess | Fever, left upper abdominal pain, left shoulder pain (Kehr sign) | Ultrasonography or CT |
| Wound botulism | Symmetric neurologic deficits, responsiveness to stimuli, blurred vision, presence of a wound | Anaerobic wound culture, serum toxin assay |
| Noninfectious | ||
| Elevated transaminase/bilirubin levels (methamphetamine) | Asymptomatic | AST, ALT, bilirubin, albumin, hepatitis serologies |
| Perforated duodenal and gastric ulcers (cocaine) | Sudden, severe abdominal pain, tachycardia, hypotension | Upright abdominal radiography, CT or US |
| Splenic infarct (cocaine) | Asymptomatic or fever and left upper abdominal pain | US or CT |
SKIN AND SOFT TISSUE COMPLICATIONS
Skin and soft tissue infections are the most common medical complication affecting PWID and the top reason for hospitalization in these patients.47 Between 6% and 32% of PWID have an active skin and soft tissue infection at any time.48,49 Risk factors include female sex, frequent injecting, infrequent skin cleaning, subcutaneous (skin popping) or intramuscular injecting because of the inability to access veins, injecting heroin compared with stimulants, HIV-positive status, and needle sharing.50
Although Staphylococcus and group A streptococci are most commonly associated with superficial skin infections, abscesses are usually polymicrobial and can contain oral flora and anaerobes.51,52 Management of skin and soft tissue infections should follow guidelines from the Infectious Diseases Society of America, which are described in a previous issue of American Family Physician.53,54 Cellulitis and abscesses without signs of systemic infection can be managed on an outpatient basis, with an awareness of the possibility of retained products in the site. Patients with signs of systemic infection should be hospitalized with appropriate sepsis management and evaluation for other sources of infection.
Delayed complications from injecting include hyperpigmentation from postinflammatory changes, scarring along vascular distributions, and deposition of foreign materials in the dermis.50 Chronic venous insufficiency affects 88% to 93% of PWID, with a higher prevalence and severity associated with increased duration of injecting.55,56
Chronic venous insufficiency and impaired lymphatic drainage can predispose PWID to venous ulceration with impaired healing of upper and lower extremities.
PULMONARY COMPLICATIONS
The annual incidence of community-acquired pneumonia is 1.4% to 2.1% in PWID, compared with 0.3% in the general population.57,58 Although the microbiology of pneumonia in PWID is largely similar to that in the general population, Staphylococcus aureus and anaerobic organisms are more common in PWID.57,58 Additionally, respiratory depression predisposes PWID to aspiration pneumonia.
Noninfectious pulmonary complications include pulmonary edema, foreign body deposition, emphysema, pulmonary embolism, and fatal asthma exacerbations. Noncardiogenic pulmonary edema can occur from opioid overdose within minutes to hours of opioid use, even if naloxone is given. Foreign body deposition within the pulmonary vasculature is common among PWID and is caused by injecting unfiltered substances or crushed pill preparations that include water-insoluble compounds such as talc, cornstarch, and cellulose that can result in granuloma formation.39,59 Patients with foreign body granulomatosis often present with progressive dyspnea on exertion, productive cough, and fatigue. In patients with foreign body granulomatosis, pulmonary function testing can show restrictive or obstructive lung patterns with occasional pulmonary hypertension, often with decreased diffusing capacity.60 Bullous emphysema and fatal asthma exacerbations are also more prevalent in PWID.61,62
CARDIOVASCULAR COMPLICATIONS
Infectious endocarditis is the most common cardiac complication associated with injection drug use. In the United States, the annual incidence of infectious endocarditis in PWID is estimated to be 1%, with a lifetime prevalence of 12%.63,64 Infectious endocarditis accounts for 5% to 15% of hospitalizations for acute infection in PWID.65
Compared with the general population, infectious endocarditis in PWID is more often caused by Staphylococcus (68% vs. 28% of cases) and more often involves the right-sided heart valves, particularly the tricuspid valve.66,67 Diagnosis of infectious endocarditis in PWID requires a higher index of suspicion because heart murmurs and peripheral signs and symptoms may not be present with right-sided infections. Although cardiac sequelae are less with right-sided infectious endocarditis, PWID have higher rates of reinfection (hazard ratio = 6.20; 95% confidence interval, 2.56 to 15.00) and valve-related complications (hazard ratio = 3.82; 95% confidence interval, 1.95 to 7.49).68
Transitioning from inpatient to outpatient treatment for infectious endocarditis may be complicated by concerns about discharging PWID with intravenous access for completion of parenteral antibiotic treatment. Patients with infectious endocarditis should initially be evaluated and stabilized in the hospital, and those with an adequate support system and home health care services and who will reliably follow up with medical visits can be discharged for completion of therapy.69 The risk of resuming unsafe injection drug use practices must be weighed against the cost of prolonged hospitalization for completion of treatment. Short-course intravenous or oral antibiotic regimens may be considered in some uncomplicated cases.69 Persons with severe valvular regurgitation from infectious endocarditis should be referred for valve replacement.
BONE AND SKELETAL INFECTIONS
Bone and skeletal infections are more common in PWID, primarily from hematogenous spread of bacteria from other sites, such as infected heart valves or skin and soft tissues. A high index of suspicion is necessary in these patients because positive blood culture and radiology findings and systemic symptoms may not be present initially, and a delay in diagnosis may result in neurologic compromise.
Most skeletal infections are caused by S. aureus and group A and G streptococci, whereas gram-negative bacilli (e.g., Pseudomonas aeruginosa) , Eikenella corrodens, and Candida are less common but increasingly reported in PWID.72–74 Tuberculosis, which is more prevalent in PWID, may also cause skeletal infections. Multiple bony sites, including vertebrae, may be involved, leading to abscess formation in the subdural or epidural spaces.
Data Sources: A comprehensive search was completed, including the Cochrane Database of Systematic Reviews, Essential Evidence Plus, and recommendations from the U.S. Preventive Services Task Force, Infectious Diseases Society of America, Centers for Disease Control and Prevention/Advisory Committee on Immunization Practices, and American Association for the Study of Liver Diseases. A PubMed search was completed using the following terms: primary care, injection drug, injection drug use, heroin injection, cocaine injection, ketamine injection, people who inject drugs. Search dates: October 2017 to March 2018.