
Am Fam Physician. 2019;99(3):145-147
Related article: Renal Cell Carcinoma: Diagnosis and Management
Author disclosure: No relevant financial affiliations.
Mr. Baker's voice was obviously hoarse on the phone. He called the office because he had been hoarse for six weeks—although he felt well otherwise. An ear, nose, and throat doctor examined Mr. Baker a few days later, removed a small tumor from his vocal cord, and the hoarseness promptly resolved.
That would have been the end of the story, except someone along the way had ordered chest radiography. The chest radiograph image was interpreted as abnormal, possibly showing a widened mediastinum. The recommended course of action was computed tomography (CT) of the chest. The chest CT scan revealed a normal mediastinum, a normal chest, and a 5-cm renal mass compatible with renal cell carcinoma.
Some doctors might argue that Mr. Baker should have had chest radiography as part of the evaluation for his hoarseness given his history of smoking and the possibility of lung cancer affecting the recurrent laryngeal nerve. I would counter that once we had found the cancer responsible for Mr. Baker's hoarseness, we did not need to go looking for a second cancer. Others might argue he should be screened for lung cancer anyway, but that's a different story altogether. A long-term follow-up study of the Mayo Lung Project has shown no reduction in lung cancer mortality from chest radiography,1 and the question of whether the benefits of CT screening outweigh its harms is still the subject of considerable debate.2,3
So, Mr. Baker called complaining of hoarseness and was given a diagnosis of kidney cancer. One had absolutely nothing to do with the other; it was just dumb luck. But was it good luck or bad luck?
Whenever I tell this story to clinical audiences, I always get the same response—laughter. They aren't laughing at Mr. Baker's diagnosis, they are laughing because they have experienced similar episodes and have faced a similar quandary. Remarkably, as pointed out in Gray and Harris's review of renal cell carcinoma in this issue of American Family Physician,4 some version of this story is now responsible for more than one-half of kidney cancer diagnoses.
You can see the effect in our national cancer statistics. Since the advent of cross-sectional imaging in the 1970s, the incidence of kidney cancer has more than doubled in the United States5 (Figure 16). The figure also shows that no corresponding change in kidney cancer mortality rates has occurred. The rising incidence and stable underlying (true) disease burden are a clear-cut case of cancer overdiagnosis: the detection of disease not destined to cause clinical illness or death.7,8 Further evidence of a stable underlying disease burden is evident in the stable rate at which patients first present with metastatic disease.
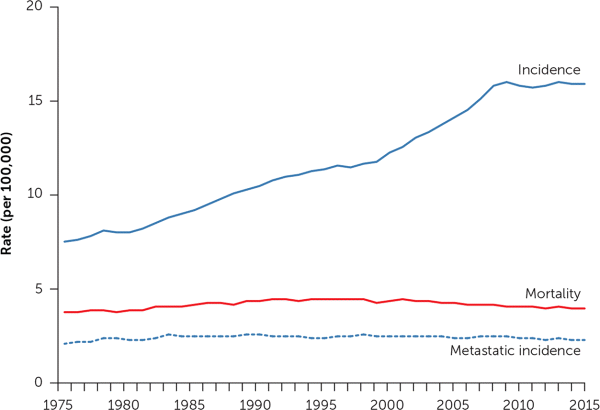
This is not an epidemic of disease, this is an epidemic of diagnosis. It is not the result of a purposeful screening effort, it is the result of unintended incidental detection—a side effect of the increasing use of cross-sectional imaging.
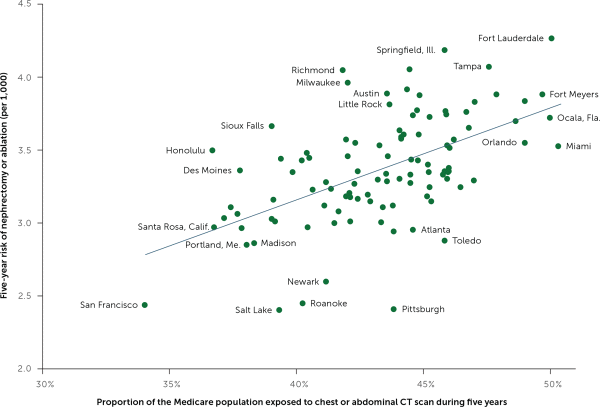
Some doctors order more CT scans than others. Similarly, the chance of being scanned is higher in some geographic regions than others. My colleagues and I found that Medicare beneficiaries residing in high scanning regions faced a higher risk of nephrectomy—undoubtedly reflecting increased incidental detection of kidney masses (Figure 2).5 The BMJ's Richard Lehman summed it up succinctly in his journal review blog, “More CTs, fewer kidneys.”9
Mr. Baker's urologist recommended a nephrectomy based on data that apparently buttressed her case. Because of early detection, the five-year survival rate for kidney cancer at that time had risen from 34% in 1950 to 62%. The current five-year survival rate is 75%.6
The five-year survival rate is rising, yet mortality is stable? How can that be right? It turns out that the combined effect of lead time (https://www.youtube.com/watch?v=ngHB1DzP5xc) and overdiagnosis bias (https://www.youtube.com/watch?v=s7QNhE59s9Q) can be very powerful. My colleagues and I investigated changes in five-year survival rates over time for 20 solid tumors. We found that increased survival was not associated with lower mortality but instead was associated with rising incidence.10 In other words, as we find more disease, the typical patient appears to do better.
Mr. Baker and I made a shared decision not to pursue surgery; however, he did undergo more CT scans. Sometimes the mass seemed to grow a bit, sometimes it seemed to shrink. About a decade later, he died of pneumonia. An autopsy showed that he had renal cell carcinoma even though he had never developed symptoms of kidney cancer, and the cancer never spread beyond his kidney. He was overdiagnosed.
I learned a great deal from Mr. Baker. Not all cancers invariably progress. Survival statistics can be very misleading, and comparisons across time and place are more reflective of diagnostic practice than the benefit of therapy. Imaging—and testing in general—has real downsides, such as stumbling onto things you wish you had not.
That is why we all need to test wisely and weigh the risks and benefits of diagnostic imaging.11 We will all stumble occasionally, and when we do, it is important to remember that sometimes the right thing to do is nothing.
