
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2019;99(3):159-165
Patient information: See related handout on gastroenteritis in children: treating dehydration, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Acute gastroenteritis is defined as a diarrheal disease of rapid onset, with or without nausea, vomiting, fever, or abdominal pain. In the United States, acute gastroenteritis accounts for 1.5 million office visits, 200,000 hospitalizations, and 300 deaths in children each year. Evaluation of a child with acute gastroenteritis should include a recent history of fluid intake and output. Significant dehydration is unlikely if parents report no decrease in oral intake or urine output and no vomiting. The physical examination is the best way to evaluate hydration status. The four-item Clinical Dehydration Scale can be used to determine severity of dehydration based on physical examination findings. In children with mild illness, stool microbiological tests are not routinely needed when viral gastroenteritis is the likely diagnosis. Mild gastroenteritis in children can be managed at home. Oral rehydration therapy, such as providing half-strength apple juice followed by the child's preferred liquids, is the mainstay of treatment for mild dehydration and is as effective as intravenous rehydration for preventing hospitalization and return to the emergency department. Oral rehydration solutions are recommended for moderate dehydration. Ondansetron may be prescribed if needed to prevent vomiting and improve tolerance of oral rehydration solutions. Hospitalization and intravenous fluids are recommended for children who do not respond to oral rehydration therapy plus an antiemetic and patients with severe dehydration (i.e., signs of shock or more than 10% dehydration). Handwashing, breastfeeding, and rotavirus vaccination reduce the incidence of acute gastroenteritis in young children.
WHAT IS NEW ON THIS TOPIC
The Clinical Dehydration Scale evaluates four clinical features to estimate degree of dehydration and is particularly useful in identifying moderate to severe dehydration. It has been validated in multiple settings in both high- and low-resource areas and compares well with assessing weight before and after rehydration, which is the standard method of evaluating for dehydration.
The WHO now recommends rehydration with a reduced osmolarity ORS. The official WHO ORS or a solution comprised of ½ teaspoon salt and 6 teaspoons sugar per 1 L water may be used.
ORS = oral rehydration solution; WHO = World Health Organization.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Oral rehydration therapy is recommended for children with mild to moderate dehydration from acute gastroenteritis. It is as effective as intravenous rehydration in preventing hospitalizations and return emergency department visits. | B | 25, 26, 28 |
| Children with mild dehydration should receive half-strength apple juice followed by preferred fluids (regular juices, milk). This approach reduces the need for eventual intravenous rehydration compared with a formal oral rehydration solution. | B | 27 |
| Handwashing with soap is an effective method for preventing episodes of gastroenteritis. Handwashing and hygiene alone, however, do not prevent rotavirus infection. | A | 37, 38, 41 |
| All children should receive an oral live, attenuated rotavirus vaccine to reduce the incidence of hospitalization, severe gastroenteritis, and death from rotavirus infection. | A | 43, 44 |
| Breastfeeding reduces the incidence of acute gastroenteritis and hospitalization from diarrheal disease in young children. | B | 46, 47 |
Worldwide, 68% of diarrheal disease occurs in young children.3 Diarrheal disease is the fifth leading cause of death in children worldwide, accounting for about 2.5 million deaths.4–6 In the United States, acute gastroenteritis is not a major cause of death but leads to significant morbidity, especially in children younger than five years, accounting for 1.5 million office visits, 200,000 hospitalizations, and 300 deaths in children each year.1,7–9
This review focuses on acute gastroenteritis in children in industrialized nations, where viruses account for 75% to 90% of childhood acute infectious gastroenteritis. Approximately 20% of cases are due to bacteria.1 Diarrhea persisting for at least 14 days is more commonly caused by parasitic infections, which account for less than 5% of acute gastroenteritis cases.1,10 The specific causative microorganisms vary with season and climate.1
Evaluation
HISTORY
The history should include onset and duration of symptoms, caregiver reports of fluid intake and output, and red flag symptoms that require aggressive treatment (Table 1).11,12 Because vomiting and diarrhea are not specific to acute gastroenteritis, other diagnoses should be considered (Table 2).1,13 Although seizures are more commonly associated with high fever, central nervous system infection, or electrolyte abnormalities, they can be caused by rotavirus infection in children.14
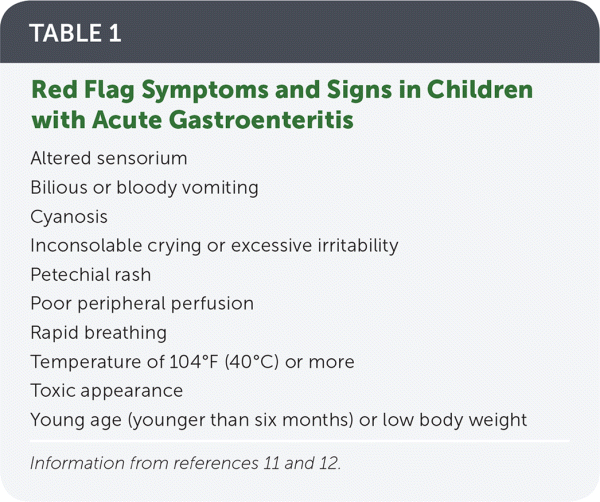
| Altered sensorium |
| Bilious or bloody vomiting |
| Cyanosis |
| Inconsolable crying or excessive irritability |
| Petechial rash |
| Poor peripheral perfusion |
| Rapid breathing |
| Temperature of 104°F (40°C) or more |
| Toxic appearance |
| Young age (younger than six months) or low body weight |
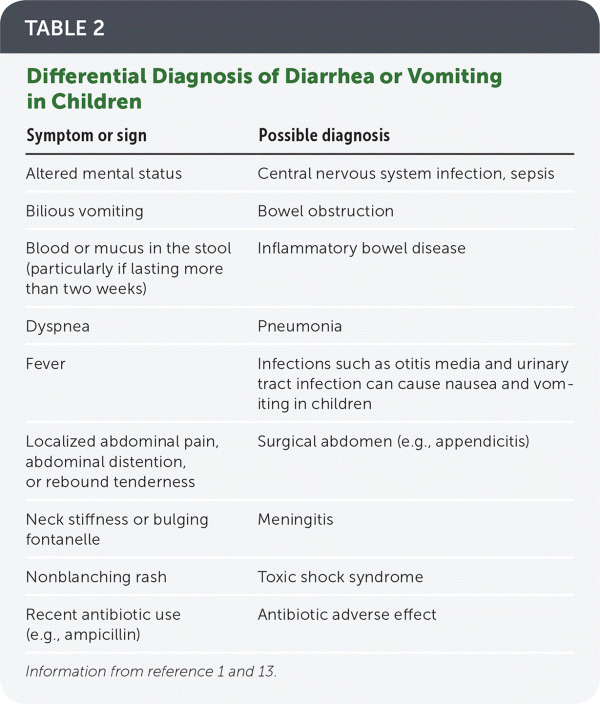
| Symptom or sign | Possible diagnosis |
|---|---|
| Altered mental status | Central nervous system infection, sepsis |
| Bilious vomiting | Bowel obstruction |
| Blood or mucus in the stool (particularly if lasting more than two weeks) | Inflammatory bowel disease |
| Dyspnea | Pneumonia |
| Fever | Infections such as otitis media and urinary tract infection can cause nausea and vomiting in children |
| Localized abdominal pain, abdominal distention, or rebound tenderness | Surgical abdomen (e.g., appendicitis) |
| Neck stiffness or bulging fontanelle | Meningitis |
| Nonblanching rash | Toxic shock syndrome |
| Recent antibiotic use (e.g., ampicillin) | Antibiotic adverse effect |
HYDRATION STATUS
Reassuring findings include normal oral intake, normal urine output, and no vomiting. If all three of these findings are present based on caregiver report, dehydration is likely not clinically significant, with a likelihood ratio approaching 0.15
However, physical examination is the best way to evaluate hydration status. The Clinical Dehydration Scale (Table 316) evaluates four clinical features to estimate degree of dehydration and is particularly useful in identifying moderate to severe dehydration. This scale has been validated in multiple settings in both high- and low-resource areas and compares well with assessing weight before vs. after rehydration.17–19
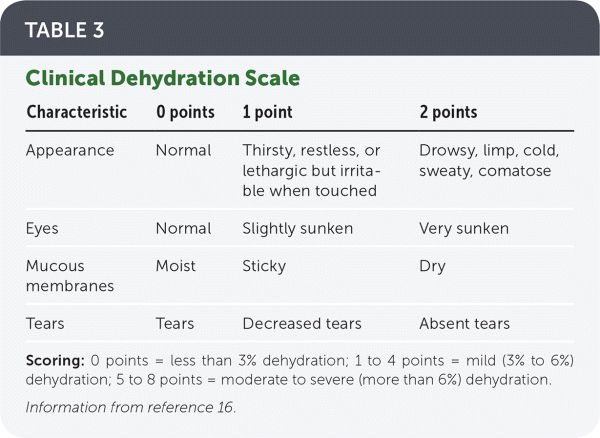
| Characteristic | 0 points | 1 point | 2 points |
|---|---|---|---|
| Appearance | Normal | Thirsty, restless, or lethargic but irritable when touched | Drowsy, limp, cold, sweaty, comatose |
| Eyes | Normal | Slightly sunken | Very sunken |
| Mucous membranes | Moist | Sticky | Dry |
| Tears | Tears | Decreased tears | Absent tears |
LABORATORY EVALUATION
The only laboratory finding useful in determining the likelihood of 5% or less dehydration is a serum bicarbonate concentration of more than 15 mEq per L (15 mmol per L; likelihood ratio = 0.18 to 0.22).20 However, electrolytes, creatinine, and glucose levels should also be ordered if intravenous rehydration will likely be needed.13 Serum sodium levels can confirm the presence of hypernatremic dehydration, which should be suspected if the patient is jittery or has hypertonia, hyperreflexia, seizures, drowsiness, or coma.
In children with mild illness, stool microbiological tests are not routinely needed when viral gastroenteritis is the likely diagnosis. However, stool studies should be obtained in patients with suspected septicemia or blood or mucus in the stool, and in those who are immunocompromised. For these patients, stool culture is the standard preferred test for identifying causative agents in bacterial gastroenteritis (antigen and/or nucleic acid amplification tests are needed to detect Clostridium difficile and Campylobacter). Polymerase chain reaction studies are increasingly being used to identify viral causes of gastroenteritis in hospitalized children because confirmation of a viral etiology can avoid unnecessary antibiotic use.21
Additional indications for stool studies include gastroenteritis after foreign travel (stool examination for ova and parasites), diarrhea that does not improve within seven days (fecal leukocytes may indicate an inflammatory cause), or symptoms occurring in the setting of a community gastroenteritis outbreak.13
Treatment
GENERAL CONSIDERATIONS
The goals of acute gastroenteritis treatment include preventing dehydration, treating dehydration when it occurs, and reducing duration and severity of symptoms.12 There are many guidelines for treating acute gastroenteritis, based largely on expert consensus. Recommendations vary among guidelines, particularly regarding dosages of oral rehydration solution (ORS).
Guidelines are consistent in recommending that children with dehydration be rehydrated, that ongoing fluid losses be replaced, that breast-feeding continue throughout rehydration, and that an age-appropriate diet be started after initial rehydration (it is not necessary to avoid milk-based products). Hospital admission is indicated for severe dehydration, social concerns (i.e., concerns about caregiver ability to follow directions for rehydration therapy or to understand which symptoms warrant a return visit), failed rehydration, or suspected serious alternative diagnoses. Evidence-based guidelines agree that antidiarrheal medications should not be used, but some guidelines recommend the antiemetic ondansetron (Zofran) as an option to improve success rates of oral rehydration.22 Recent evidence suggests that clinical pathway tools/algorithms also help increase the use of oral rehydration and decrease the use of intravenous fluids and the length of emergency department stay.18,23
MILD DEHYDRATION (6% OR LESS)
Mild dehydration from acute gastroenteritis can be managed at home, with oral rehydration therapy as the mainstay of treatment.24 A meta-analysis found no significant difference in hospitalizations or return emergency department visits between oral and intravenous rehydration,25 and only one out of 25 children treated with an ORS will eventually require intravenous fluids.26
The specific electrolyte composition of the ORS is not important for mild dehydration. For example, one study of children older than six months showed that half-strength apple juice followed by preferred fluids (regular juices, milk) reduced the need for eventual intravenous rehydration compared with a formal ORS,27 most likely because children were more apt to drink the preferred fluids than the ORS. After each loose stool, the World Health Organization (WHO) recommends giving children younger than two years 50 to 100 mL of fluid and children two to 10 years of age 100 to 200 mL of fluid; older children may have as much fluid as they want. Children may consume up to 20 mL per kg of body weight per hour.12
MODERATE TO SEVERE DEHYDRATION (MORE THAN 6%)
Treatment of moderate dehydration includes an ORS plus medication if needed to decrease vomiting and improve tolerance of the ORS. For children with moderate dehydration, oral rehydration is as effective as intravenous rehydration in preventing hospitalization and return visits.28
ORS. In a recent change, WHO now recommends its reduced osmolarity ORS, which contains 75 mEq per L of sodium and 75 mmol per L of glucose dissolved in 1 L of water.12 Previously, the standard WHO ORS contained 90 mEq per L of sodium. If using this older solution in infants younger than six months, an additional 100 to 200 mL of clean water should be added. Alternatively, a homemade solution of ½ teaspoon salt and 6 teaspoons sugar in 1 L of water may be used.12 Table 4 includes WHO guidelines for administering ORS in children.12
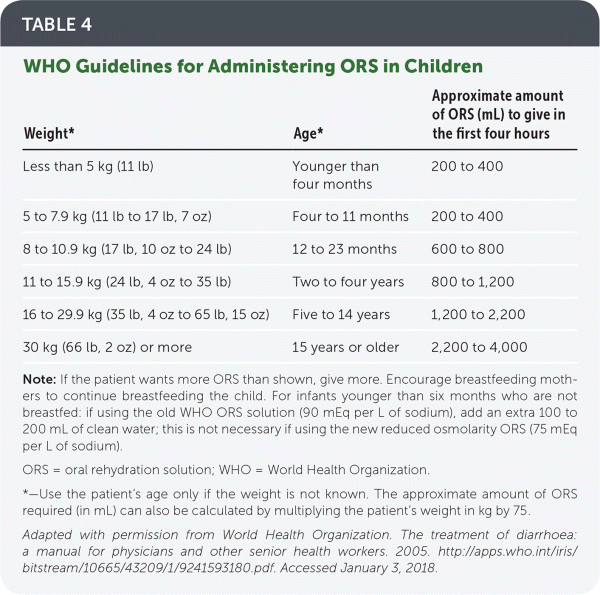
| Weight* | Age* | Approximate amount of ORS (mL) to give in the first four hours |
|---|---|---|
| Less than 5 kg (11 lb) | Younger than four months | 200 to 400 |
| 5 to 7.9 kg (11 lb to 17 lb, 7 oz) | Four to 11 months | 400 to 600 [corrected] |
| 8 to 10.9 kg (17 lb, 10 oz to 24 lb) | 12 to 23 months | 600 to 800 |
| 11 to 15.9 kg (24 lb, 4 oz to 35 lb) | Two to four years | 800 to 1,200 |
| 16 to 29.9 kg (35 lb, 4 oz to 65 lb, 15 oz) | Five to 14 years | 1,200 to 2,200 |
| 30 kg (66 lb, 2 oz) or more | 15 years or older | 2,200 to 4,000 |
Caregivers should be taught how to give ORS via syringe to newborns and via a spoon or cup to older infants and young children. Children younger than two years should be given 1 teaspoon every one to two minutes; older children should be encouraged to take frequent sips directly from the cup. If vomiting occurs, the recommendation is to wait five to 10 minutes and then start offering the ORS again more slowly, every two to three minutes.12
Antiemetics. Ondansetron is commonly used when needed to prevent vomiting while drinking the ORS. In a Cochrane review, children who received an antiemetic were less likely to need intravenous rehydration (relative risk = 0.40; 95% confidence interval [CI], 0.26 to 0.60).29 Another systematic review showed that ondansetron decreased the number of hospitalizations (number needed to treat [NNT] = 14; 95% CI, 9 to 44), decreased the need for intravenous rehydration (NNT = 5; 95% CI, 4 to 8), and decreased further vomiting (NNT = 5; 95% CI, 4 to 7), but it was associated with increased risk of diarrhea.30
The typical dose of ondansetron is 2 mg for children weighing 8 to 15 kg (17 lb, 10 oz to 33 lb), 4 mg for children weighing 15 to 30 kg (33 lb to 66 lb, 2 oz), and 8 mg for children weighing more than 30 kg. The dose may be repeated if the child vomits within 15 minutes of taking the medication. Ondansetron should be avoided in patients with congenital long QT syndrome. In addition, electrolytes should always be assessed in children with severe dehydration before administration because hypomagnesemia and hypokalemia increase the risk of QT prolongation. [corrected] Older antiemetics, such as promethazine and metoclopramide (Reglan), have higher rates of adverse reactions and are not generally recommended.25,31
Intravenous Rehydration. Patients who do not respond to oral rehydration therapy plus an antiemetic and patients with severe dehydration (i.e., signs of clinical shock or more than 10% dehydration) require hospitalization and intravenous rehydration.12 Table 5 includes WHO guidelines for intravenous treatment of severe dehydration.12 Children who can drink should be given ORS with a syringe, spoon, or cup until the intravenous infusion is established. In resource-limited settings, nasogastric rehydration may be considered if intravenous access is impractical.19

| Start IV fluids immediately; if the patient can drink, give oral rehydration solution until the IV infusion is established; give 100 mL per kg of Ringer solution* divided as follows: |
| Reassess the patient every one to two hours; if hydration is not improving, give the IV drip more rapidly. |
| After six hours (infants) or three hours (older patients), assess the patient to determine the next steps in treatment. |
Nitazoxanide. In one small study conducted in Egypt, children with severe rotavirus-related diarrhea who received nitazoxanide (Alinia) demonstrated a significant reduction in illness duration. Although it is an antiparasitic, in-vitro studies have shown that nitazoxanide inhibits replication of a broad range of viruses.32 Larger studies are needed before the use of nitazoxanide is widely incorporated into practice.
Supplements. Although Lactobacillus has been studied as a treatment for hospitalized patients receiving oral rehydration therapy, its benefit is unclear. One meta-analysis found that it only slightly decreased duration of illness and stool frequency.33 The WHO recommends zinc supplementation during acute diarrhea based on studies conducted primarily in countries where children are likely to be zinc deficient.12 A Cochrane review found insufficient data to determine if zinc supplementation improves outcomes.34
Prevention
Improving sanitation and water quality are important for reducing diarrheal disease in low-income countries.35,36 A Cochrane review found that point-of-use water purification methods, specifically the use of ceramic and biosand filters (i.e., column devices with a biofilm top and layers of sand and gravel), reduce the incidence of diarrhea by one-half.35 These measures are generally not needed in the United States and other high-income countries.
HANDWASHING
Handwashing is recommended as the primary means of preventing gastroenteritis. A meta-analysis of 30 studies showed that handwashing campaigns reduce the incidence of gastrointestinal infections by 30% (95% CI, 19 to 43).37 A Cochrane review found similar results.38 There is no evidence that antibacterial soaps work better than nonantibacterial soaps.37 The use of alcohol-based hand sanitizers in addition to standard handwashing education can reduce gastroenteritis in offices and day cares and can decrease school absenteeism by 30%.39,40 Handwashing and hygiene alone, however, do not prevent rotavirus infection.41
VACCINES
Before the availability of a rotavirus vaccine, nearly all children worldwide experienced a rotavirus infection by three to five years of age. However, the rate of rotavirus infection has been markedly reduced with use of the vaccine. Postmarketing studies in Mexico and Brazil have shown that the vaccine prevents 80,000 hospitalizations and 1,300 diarrheal deaths per year.42
All children should receive an oral live, attenuated rotavirus vaccine, which can be initiated between six and 15 weeks of life, to reduce the risk of severe gastroenteritis, hospitalization, and death from rotavirus infection.43,44 The two rotavirus vaccines approved for use in the United States are Rotateq (recommended at two, four, and six months of age) and Rotarix (recommended at two and four months of age). The minimal dosing interval is four weeks, and all vaccines should be given by eight months of age. Preterm infants should be vaccinated using the routine schedule.44
A norovirus vaccine is currently undergoing clinical trials. Studies have shown a robust immune response and good tolerability in adults and children.45
OTHER PREVENTIVE METHODS
Exclusive breastfeeding for four months and partial breast-feeding thereafter are associated with lower rates of acute gastroenteritis in the first year of life46 and decreased rates of hospitalization from diarrheal disease.47 A cohort study estimated that 53% of diarrheal hospitalizations could be prevented each month with exclusive breastfeeding, with sustained effects after breastfeeding cessation.47
A great deal of research has focused on the immunologic properties of human milk. Antibodies in human milk offer a small part of the infant's immune protection, with the intestinal microbiome, prebiotics, probiotics, mucosal immunity, nucleotides, and oligosaccharides having greater roles.48 Epidermal growth factor in breast milk induces intestinal epithelium maturation, immunoglobulin A, and oligosaccharides, which prevent pathogen attachment, and lactoferrin in breast milk offers antimicrobial properties.46,48
Some evidence demonstrates that daily administration of probiotics to children in day care reduces the incidence of acute infectious diarrhea without adverse effects. For example, one study showed that children in day care centers that administer Lactobacillus reuteri have reduced health care costs and episodes of diarrhea.49 Evidence is mixed, however, in most studies of day care centers that add probiotics to milk-based feedings.50–52
More studies are needed to determine which specific strains and dosages of probiotics are most helpful. Family physicians may wish to discuss with parents the potential benefit of probiotics for prevention of infectious gastroenteritis in children.50
This article updates previous articles on this topic by Churgay and Aftab,53 ,54 Burkhart,55 and Eliason and Lewan.56
Data Sources: An initial literature search was performed using Essential Evidence Plus and PubMed. This was supplemented by searches of the Cochrane database and National Guideline Clearinghouse. Key words included rotavirus, gastroenteritis, dehydration, diarrheal disease, diarrhea, nausea, and vomiting. Search dates: October 1, 2017, to May 28, 2018.
