Am Fam Physician. 2019;99(4):237-243
Author disclosure: No relevant financial affiliations.
Several recent large randomized controlled trials have significantly improved physicians' knowledge about the impact of medications for type 2 diabetes mellitus on patient-oriented outcomes. A concise and organized way to evaluate pharmacotherapy options is to use the five patient-oriented STEPS criteria: safety, tolerability, effectiveness, price, and simplicity. The first-line treatment option, metformin, is safe and fairly well-tolerated, has excellent long-term effectiveness on patient-oriented outcomes, is moderately priced, and has a simple dosing regimen. However, most patients with type 2 diabetes require more than one medication. The STEPS approach can help choose subsequent medications if metformin does not provide adequate glycemic control.
Only a few years ago, lifestyle modification, sulfonylureas, metformin, and insulin were the only treatment options for type 2 diabetes mellitus. Now, family physicians have approximately 40 medications in 10 categories to manage hyperglycemia in patients with type 2 diabetes. However, the availability of so many choices makes therapeutic decisions more complex. Although all 40 medications will improve blood glucose levels, that is not sufficient. As family physicians, we seek to treat the whole person, not just blood glucose levels, insulin resistance, and islet cell dysfunction. Our patients with diabetes depend on us to help reduce their long-term risk of myocardial infarction, stroke, amputation, dialysis, and premature mortality.
Several recent large randomized controlled trials have significantly improved our knowledge about the impact of diabetes medications on patient-oriented outcomes. After the thiazolidinedione (TZD) rosiglitazone (Avandia) was found to increase the risk of myocardial infarction,1 the U.S. Food and Drug Administration required newly approved diabetes drugs to undergo rigorous postmarketing studies of long-term cardiovascular harm.2 Studies evaluating harms, such as major cardiovascular events and cardiovascular mortality, also have the potential to show us which agents confer long-term benefits for those outcomes. The results of these studies can be used to make better choices for our patients with type 2 diabetes.
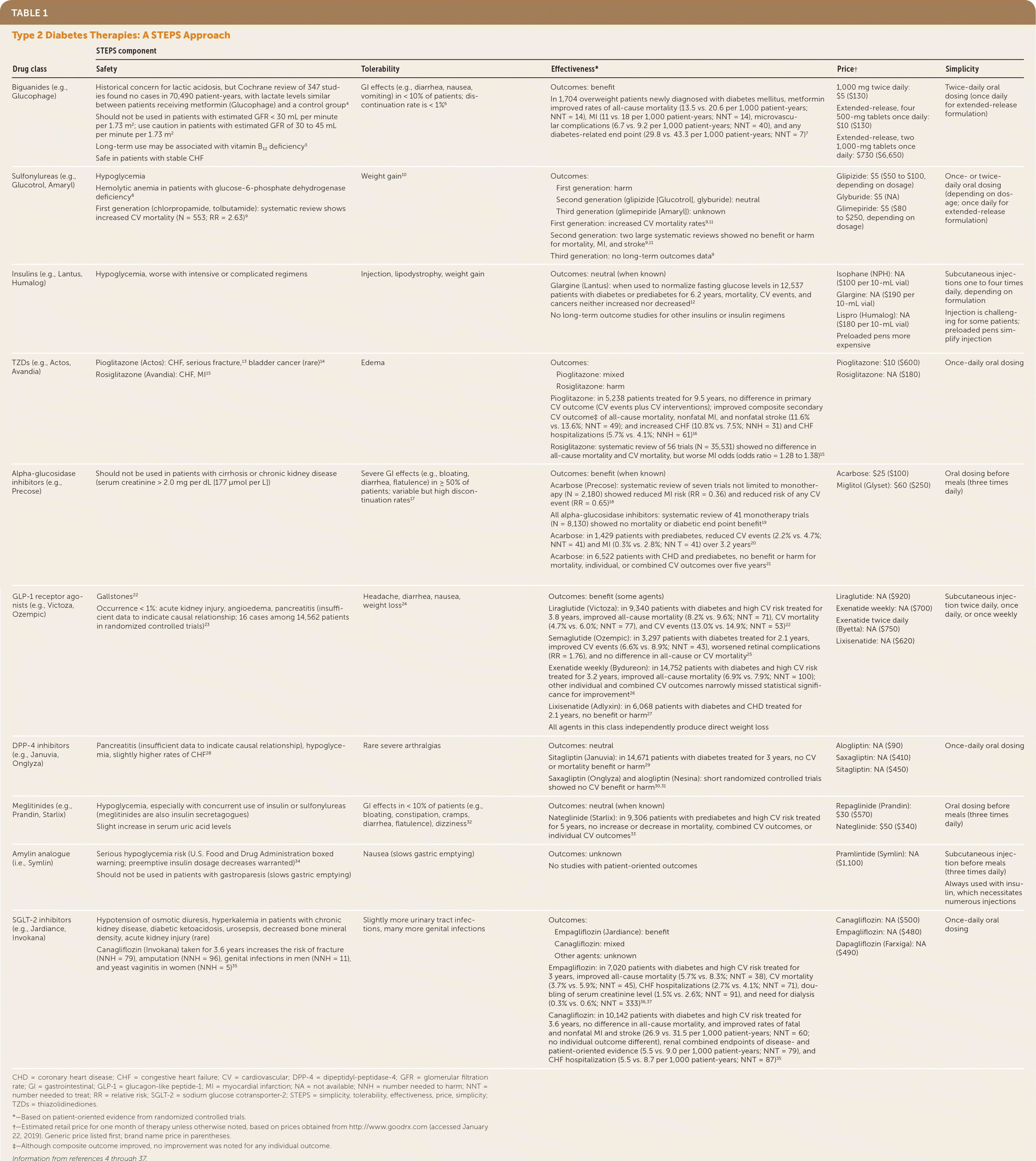
A concise and organized way to evaluate pharmacotherapy options for diabetes is to use the five patient-oriented STEPS criteria: safety, tolerability, effectiveness, price, and simplicity.3 Table 1 presents the STEPS approach for each category of diabetes medication.4–37 It permits side-by-side comparisons of the pros and cons, and reveals some insights for clinical decision making.
| Drug class | STEPS component | ||||
|---|---|---|---|---|---|
| Safety | Tolerability | Effectiveness* | Price† | Simplicity | |
| Biguanides (e.g., Glucophage) | Historical concern for lactic acidosis, but Cochrane review of 347 studies found no cases in 70,490 patient-years, with lactate levels similar between patients receiving metformin (Glucophage) and a control group4 Should not be used in patients with estimated GFR <30 mL per minute per 1.73 m2; use caution in patients with estimated GFR of 30 to 45 mL per minute per 1.73 m2 Long-term use may be associated with vitamin B12 deficiency5 Safe in patients with stable CHF | GI effects (e.g., diarrhea, nausea, vomiting) in <10% of patients; discontinuation rate is <1%6 | Outcomes: benefit In 1,704 overweight patients newly diagnosed with diabetes mellitus, metformin improved rates of all-cause mortality (13.5 vs. 20.6 per 1,000 patient-years; NNT = 14), MI (11 vs. 18 per 1,000 patient-years; NNT = 14), microvascular complications (6.7 vs. 9.2 per 1,000 patient-years; NNT = 40), and any diabetes-related end point (29.8 vs. 43.3 per 1,000 patient-years; NNT = 7)7 | 1,000 mg twice daily: $5 ($130) Extended-release, four 500-mg tablets once daily: $10 ($130) Extended-release, two 1,000-mg tablets once daily: $730 ($6,650) | Twice-daily oral dosing (once daily for extended-release formulation) |
| Sulfonylureas (e.g., Glucotrol, Amaryl) | Hypoglycemia Hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency8 First generation (chlorpropamide, tolbutamide): systematic review shows increased CV mortality (N = 553; RR = 2.63)9 | Weight gain10 | Outcomes: • First generation: harm • Second generation (glipizide [Glucotrol], glyburide): neutral • Third generation (glimepiride [Amaryl]): unknown First generation: increased CV mortality rates9,11 Second generation: two large systematic reviews showed no benefit or harm for mortality, MI, and stroke9,11 Third generation: no long-term outcomes data9 | Glipizide: $5 ($50 to $100, depending on dosage) Glyburide: $5 (NA) Glimepiride: $5 ($80 to $250, depending on dosage) | Once- or twice-daily oral dosing (depending on dosage; once daily for extended-release formulation) |
| Insulins (e.g., Lantus, Humalog) | Hypoglycemia, worse with intensive or complicated regimens | Injection, lipodystrophy, weight gain | Outcomes: neutral (when known) Glargine (Lantus): when used to normalize fasting glucose levels in 12,537 patients with diabetes or prediabetes for 6.2 years, mortality, CV events, and cancers neither increased nor decreased12 No long-term outcome studies for other insulins or insulin regimens | Isophane (NPH): NA ($100 per 10-mL vial) Glargine: NA ($190 per 10-mL vial) Lispro (Humalog): NA ($180 per 10-mL vial) Preloaded pens more expensive | Subcutaneous injections one to four times daily, depending on formulation Injection is challenging for some patients; preloaded pens simplify injection |
| TZDs (e.g., Actos, Avandia) | Pioglitazone (Actos): CHF, serious fracture,13 bladder cancer (rare)14 Rosiglitazone (Avandia): CHF, MI15 | Edema | Outcomes: •Pioglitazone: mixed •Rosiglitazone: harm Pioglitazone: in 5,238 patients treated for 9.5 years, no difference in primary CV outcome (CV events plus CV interventions); improved composite secondary CV outcome‡ of all-cause mortality, nonfatal MI, and nonfatal stroke (11.6% vs. 13.6%; NNT = 49); and increased CHF (10.8% vs. 7.5%; NNH = 31) and CHF hospitalizations (5.7% vs. 4.1%; NNH = 61)16 Rosiglitazone: systematic review of 56 trials (N = 35,531) showed no difference in all-cause mortality and CV mortality, but worse MI odds (odds ratio = 1.28 to 1.38)15 | Pioglitazone: $10 ($600) Rosiglitazone: NA ($180) | Once-daily oral dosing |
| Alpha-glucosidase inhibitors (e.g., Precose) | Should not be used in patients with cirrhosis or chronic kidney disease (serum creatinine <2.0 mg per dL [177 μmol per L]) | Severe GI effects (e.g., bloating, diarrhea, flatulence) in ≥50% of patients; variable but high discontinuation rates17 | Outcomes: benefit (when known) Acarbose (Precose): systematic review of seven trials not limited to monotherapy (N = 2,180) showed reduced MI risk (RR = 0.36) and reduced risk of any CV event (RR = 0.65)18 All alpha-glucosidase inhibitors: systematic review of 41 monotherapy trials (N = 8,130) showed no mortality or diabetic end point benefit19 Acarbose: in 1,429 patients with prediabetes, reduced CV events (2.2% vs. 4.7%; NNT = 41) and MI (0.3% vs. 2.8%; NN T = 41) over 3.2 years20 Acarbose: in 6,522 patients with CHD and prediabetes, no benefit or harm for mortality, individual, or combined CV outcomes over five years21 | Acarbose: $25 ($100) Miglitol (Glyset): $60 ($250) | Oral dosing before meals (three times daily) |
| GLP-1 receptor agonists (e.g., Victoza, Ozempic) | Gallstones22 Occurrence <1%: acute kidney injury, angioedema, pancreatitis (insufficient data to indicate causal relationship; 16 cases among 14,562 patients in randomized controlled trials)23 | Headache, diarrhea, nausea, weight loss24 | Outcomes: benefit (some agents) Liraglutide (Victoza): in 9,340 patients with diabetes and high CV risk treated for 3.8 years, improved all-cause mortality (8.2% vs. 9.6%; NNT = 71), CV mortality (4.7% vs. 6.0%; NNT = 77), and CV events (13.0% vs. 14.9%; NNT = 53)22 Semaglutide (Ozempic): in 3,297 patients with diabetes treated for 2.1 years, improved CV events (6.6% vs. 8.9%; NNT = 43), worsened retinal complications (RR = 1.76), and no difference in all-cause or CV mortality 25 Exenatide weekly (Bydureon): in 14,752 patients with diabetes and high CV risk treated for 3.2 years, improved all-cause mortality (6.9% vs. 7.9%; NNT = 100); other individual and combined CV outcomes narrowly missed statistical significance for improvement26 Lixisenatide (Adlyxin): in 6,068 patients with diabetes and CHD treated for 2.1 years, no benefit or harm27 All agents in this class independently produce direct weight loss | Liraglutide: NA ($920) Exenatide weekly: NA ($700) Exenatide twice daily (Byetta): NA ($750) Lixisenatide: NA ($620) | Subcutaneous injection twice daily, once daily, or once weekly |
| DPP-4 inhibitors (e.g., Januvia, Onglyza) | Pancreatitis (insufficient data to indicate causal relationship), hypoglycemia, slightly higher rates of CHF28 | Rare severe arthralgias | Outcomes: neutral Sitagliptin (Januvia): in 14,671 patients with diabetes treated for 3 years, no CV or mortality benefit or harm29 Saxagliptin (Onglyza) and alogliptin (Nesina): short randomized controlled trials showed no CV benefit or harm30,31 | Alogliptin: NA ($90) Saxagliptin: NA ($410) Sitagliptin: NA ($450) | Once-daily oral dosing |
| Meglitinides (e.g., Prandin, Starlix) | Hypoglycemia, especially with concurrent use of insulin or sulfonylureas (meglitinides are also insulin secretagogues) Slight increase in serum uric acid levels | GI effects in <10% of patients (e.g., bloating, constipation, cramps, diarrhea, flatulence), dizziness32 | Outcomes: neutral (when known) Nateglinide (Starlix): in 9,306 patients with prediabetes and high CV risk treated for 5 years, no increase or decrease in mortality, combined CV outcomes, or individual CV outcomes33 | Repaglinide (Prandin): $30 ($570) Nateglinide: $50 ($340) | Oral dosing before meals (three times daily) |
| Amylin analogue (i.e., Symlin) | Serious hypoglycemia risk (U.S. Food and Drug Administration boxed warning; preemptive insulin dosage decreases warranted)34 Should not be used in patients with gastroparesis (slows gastric emptying) | Nausea (slows gastric emptying) | Outcomes: unknown No studies with patient-oriented outcomes | Pramlintide (Symlin): NA ($1,100) | Subcutaneous injection before meals (three times daily) Always used with insulin, which necessitates numerous injections |
| SGLT-2 inhibitors (e.g., Jardiance, Invokana) | Hypotension of osmotic diuresis, hyperkalemia in patients with chronic kidney disease, diabetic ketoacidosis, urosepsis, decreased bone mineral density, acute kidney injury (rare) Canagliflozin (Invokana) taken for 3.6 years increases the risk of fracture (NNH = 79), amputation (NNH = 96), genital infections in men (NNH = 11), and yeast vaginitis in women (NNH = 5)35 | Slightly more urinary tract infections, many more genital infections | Outcomes: • Empagliflozin (Jardiance): benefit • Canagliflozin: mixed • Other agents: unknown Empagliflozin: in 7,020 patients with diabetes and high CV risk treated for 3 years, improved all-cause mortality (5.7% vs. 8.3%; NNT = 38), CV mortality (3.7% vs. 5.9%; NNT = 45), CHF hospitalizations (2.7% vs. 4.1%; NNT = 71), doubling of serum creatinine level (1.5% vs. 2.6%; NNT = 91), and need for dialysis (0.3% vs. 0.6%; NNT = 333)36,37 Canagliflozin: in 10,142 patients with diabetes and high CV risk treated for 3.6 years, no difference in all-cause mortality, and improved rates of fatal and nonfatal MI and stroke (26.9 vs. 31.5 per 1,000 patient-years; NNT = 60; no individual outcome different), renal combined endpoints of disease- and patient-oriented evidence (5.5 vs. 9.0 per 1,000 patient-years; NNT = 79), and CHF hospitalization (5.5 vs. 8.7 per 1,000 patient-years; NNT = 87)35 | Canagliflozin: NA ($500) Empagliflozin: NA ($480) Dapagliflozin (Farxiga): NA ($490) | Once-daily oral dosing |
The American Diabetes Association recommends the biguanide metformin (Glucophage) as first-line pharmacotherapy for type 2 diabetes.38 The STEPS criteria show why: it is safe and fairly well-tolerated, has excellent long-term effectiveness on patient-oriented outcomes, is moderately priced, and has a simple dosing regimen. No other diabetes medication excels in the STEPS criteria as well as metformin. However, most patients with type 2 diabetes require more than one medication. The STEPS approach can help us choose subsequent medications if metformin does not provide adequate glycemic control for our patients.
Focusing first on effectiveness, the table shows evidence of improved patient-oriented outcomes from glucagon-like peptide-1 (GLP-1) receptor agonists and sodium glucose cotransporter-2 (SGLT-2) inhibitors in patients who are at high cardiovascular risk or have known cardiovascular disease.22,36,37 Such improvements are likely to be significantly more modest in patients who are not at high cardiovascular risk. No long-term improvement in patient-oriented outcomes has been demonstrated for the dipeptidylpeptidase-4 (DPP-4) inhibitors or for the amylin analogue pramlintide (Symlin). The high prices and lack of favorable long-term outcomes leave the use of agents such as sitagliptin (Januvia) and pramlintide hard to justify. The alpha-glucosidase inhibitor acarbose (Precose) has evidence to support improved patient-oriented outcomes,18 and as with metformin, its price is quite low.
Focusing next on price, the table shows that metformin and, to a lesser extent, acarbose have evidence of a favorable effect on long-term outcomes and are relatively inexpensive. Likewise, inexpensive generic pioglitazone (Actos) is an option with a mix of potential benefits and harms.16 Sulfonylureas and older insulins are also inexpensive, and although there is no evidence from randomized trials of long-term patient-oriented benefits, there is also no evidence of long-term end-organ harm.9,11,12
Focusing next on safety, the table shows that several agents are concerning because they increase hypoglycemia risk (e.g., sulfonylureas, insulins, meglitinides, pramlintide) or require monitoring, dose adjustments, or discontinuation in patients with chronic kidney disease (e.g., metformin, acarbose).
Focusing last on simplicity, the table shows that several agents may be challenging for some patients because of the need for frequent or complicated dosing or injections. Poor memory, eyesight, or health literacy may make such a challenging regimen a poor option for some patients.
Although agents within a drug category are more similar than different, a STEPS table comparing each insulin would highlight differences in safety, price, and simplicity. A table comparing each GLP-1 receptor agonist would show that only some (liraglutide [Victoza], semaglutide [Ozempic], exenatide) have proven cardiovascular benefit,22,25,26 whereas others have no evidence of benefit and still others have outcome studies that have not been completed.39,40
Major guidelines are increasingly aligned with a STEPS approach to pharmacotherapy for type 2 diabetes. Although it has traditionally focused on disease-oriented outcomes, the American Diabetes Association was more patient-oriented in 2018, strongly recommending metformin as first-line therapy, with additional agents added after consideration of hypoglycemia risk, comorbidities, potential adverse effects, effectiveness, price, and delivery method.41 The American Academy of Family Physicians–endorsed 2017 guideline on pharmacotherapy for type 2 diabetes from the American College of Physicians shares an emphasis on safety, tolerability, effectiveness, and price when making initial choices about monotherapy and combination therapy, while focusing on improving patient-oriented outcomes amidst a dearth of evidence regarding specific combinations of hypoglycemic drugs.42 Likewise, the 2017 guideline from the Department of Veterans Affairs and Department of Defense also recommends metformin first, then additional agents according to evidence of effectiveness and consideration of safety, adverse effects, cost, and comorbidities.43 All of these guidelines are consistent with an evidence-based STEPS approach.
Data Sources: Essential Evidence Plus and the Cochrane database were searched using the keywords type 2 diabetes and medication therapy. Trials and systematic reviews referenced in the 2017 American Diabetes Association guideline and package inserts of newer medications, and selected relevant references were used. Search dates: August 2017 to October 2018.