
Am Fam Physician. 2019;99(5):330-333
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the comparative effectiveness and harms of treatments for uterine fibroids, and what is the risk of finding unexpected leiomyosarcoma in women with fibroids?
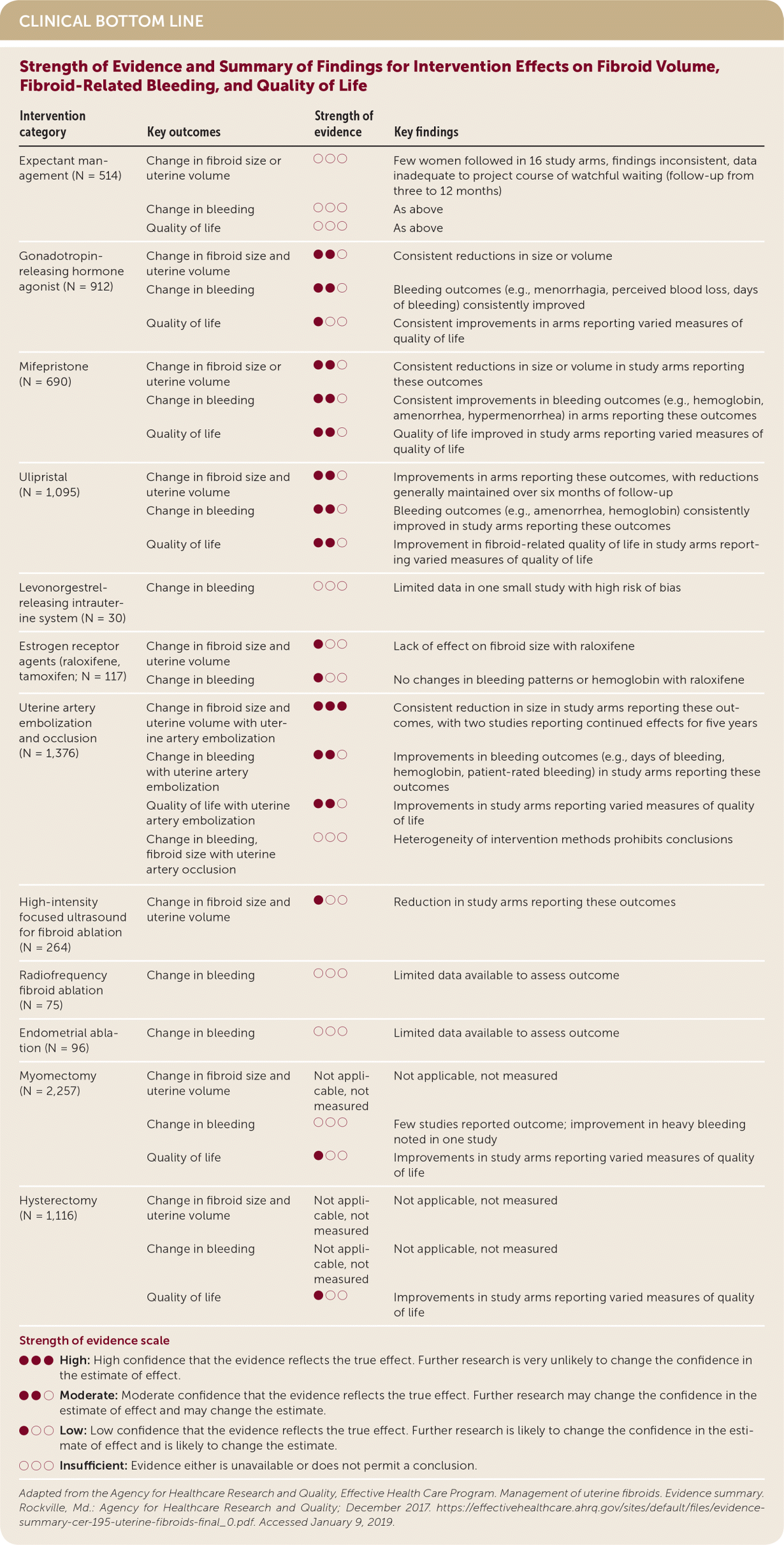
| Intervention category | Key outcomes | Strength of evidence | Key findings |
|---|---|---|---|
| Expectant management (N = 514) | Change in fibroid size or uterine volume | ○○○ | Few women followed in 16 study arms, findings inconsistent, data inadequate to project course of watchful waiting (follow-up from three to 12 months) |
| Change in bleeding | ○○○ | As above | |
| Quality of life | ○○○ | As above | |
| Gonadotropin-releasing hormone agonist (N = 912) | Change in fibroid size and uterine volume | ●●○ | Consistent reductions in size or volume |
| Change in bleeding | ●●○ | Bleeding outcomes (e.g., menorrhagia, perceived blood loss, days of bleeding) consistently improved | |
| Quality of life | ●○○ | Consistent improvements in arms reporting varied measures of quality of life | |
| Mifepristone (N = 690) | Change in fibroid size or uterine volume | ●●○ | Consistent reductions in size or volume in study arms reporting these outcomes |
| Change in bleeding | ●●○ | Consistent improvements in bleeding outcomes (e.g., hemoglobin, amenorrhea, hypermenorrhea) in arms reporting these outcomes | |
| Quality of life | ●●○ | Quality of life improved in study arms reporting varied measures of quality of life | |
| Ulipristal (N = 1,095) | Change in fibroid size and uterine volume | ●●○ | Improvements in arms reporting these outcomes, with reductions generally maintained over six months of follow-up |
| Change in bleeding | ●●○ | Bleeding outcomes (e.g., amenorrhea, hemoglobin) consistently improved in study arms reporting these outcomes | |
| Quality of life | ●●○ | Improvement in fibroid-related quality of life in study arms reporting varied measures of quality of life | |
| Levonorgestrel-releasing intrauterine system (N = 30) | Change in bleeding | ○○○ | Limited data in one small study with high risk of bias |
| Estrogen receptor agents (raloxifene, tamoxifen; N = 117) | Change in fibroid size and uterine volume | ●○○ | Lack of effect on fibroid size with raloxifene |
| Change in bleeding | ●○○ | No changes in bleeding patterns or hemoglobin with raloxifene | |
| Uterine artery embolization and occlusion (N = 1,376) | Change in fibroid size and uterine volume with uterine artery embolization | ●●● | Consistent reduction in size in study arms reporting these outcomes, with two studies reporting continued effects for five years |
| Change in bleeding with uterine artery embolization | ●●○ | Improvements in bleeding outcomes (e.g., days of bleeding, hemoglobin, patient-rated bleeding) in study arms reporting these outcomes | |
| Quality of life with uterine artery embolization | ●●○ | Improvements in study arms reporting varied measures of quality of life | |
| Change in bleeding, fibroid size with uterine artery occlusion | ○○○ | Heterogeneity of intervention methods prohibits conclusions | |
| High-intensity focused ultrasound for fibroid ablation (N = 264) | Change in fibroid size and uterine volume | ●○○ | Reduction in study arms reporting these outcomes |
| Radiofrequency fibroid ablation (N = 75) | Change in bleeding | ○○○ | Limited data available to assess outcome |
| Endometrial ablation (N = 96) | Change in bleeding | ○○○ | Limited data available to assess outcome |
| Myomectomy (N = 2,257) | Change in fibroid size and uterine volume | Not applicable, not measured | Not applicable, not measured |
| Change in bleeding | ○○○ | Few studies reported outcome; improvement in heavy bleeding noted in one study | |
| Quality of life | ●○○ | Improvements in study arms reporting varied measures of quality of life | |
| Hysterectomy (N = 1,116) | Change in fibroid size and uterine volume | Not applicable, not measured | Not applicable, not measured |
| Change in bleeding | Not applicable, not measured | Not applicable, not measured | |
| Quality of life | ●○○ | Improvements in study arms reporting varied measures of quality of life |
Evidence-Based Answer
Medical therapy (gonadotropin-releasing hormone [GnRH] agonists, mifepristone, ulipristal) or uterine artery embolization (UAE) reduces fibroid size (Strength of Recommendation [SOR]: C, based on disease-oriented evidence), reduces bleeding (SOR: A, based on consistent, good-quality patient-oriented evidence), and improves fibroid-related quality of life (SOR: A, based on consistent, good-quality patient-oriented evidence). High-intensity focused ultrasound reduces fibroid size, but its effect on quality of life is unknown. (SOR: C, based on disease-oriented evidence.) Myomectomy and hysterectomy improve quality of life. (SOR: A, based on consistent, good-quality patient-oriented evidence.) The risk that a uterine mass that is believed to be a fibroid is actually a leiomyosarcoma ranges from fewer than one to as many as 13 per 10,000 women who opted for surgical management.1
Practice Pointers
Uterine fibroids are benign smooth muscle tumors of the uterus. They are estimated to affect 26 million women between 15 and 50 years of age. A 2012 survey estimated that annual health care costs associated with treatment of uterine fibroids exceed $9.4 billion annually.2
A variety of treatments are available, including medical therapy and nonsurgical and surgical interventions. This Agency for Healthcare Research and Quality (AHRQ) review assessed effectiveness of each intervention based on fibroid characteristics, symptoms, quality of life, and patient satisfaction.
The review found consistent evidence that UAE is effective for reducing fibroid size and uterine volume. There was moderate strength of evidence that GnRH agonists, mifepristone, and ulipristal also reduce fibroid size. There was low strength of evidence, primarily because of poor study design, that high-intensity focused ultrasound decreases the size of fibroids.
Bleeding was the most common symptom reported in the studies reviewed. There was moderate strength of evidence that mifepristone, ulipristal, and UAE reduce bleeding. Evidence was insufficient to determine the effect of myomectomy or the levonorgestrel-releasing intrauterine system on bleeding patterns.
There was moderate strength of evidence that mifepristone, ulipristal, and UAE improve quality of life, and there was low strength of evidence that GnRH agonists, myomectomy, and hysterectomy improve quality of life. Because of limited data, the effectiveness of the treatment options could not be directly compared.
There is a lack of evidence that patient and fibroid characteristics can be used to predict risk of leiomyosarcoma. For every 10,000 patients who opt for surgical management of fibroids, fewer than one to as many as 13 women may be found to have leiomyosarcoma.
There are four main goals in the treatment of uterine fibroids: improving symptoms, reducing fibroid size and sustaining the decreased size, preserving fertility if desired, and avoiding harm.3 Other factors influencing treatment choice include fibroid size and location, age of the patient, and access to treatment. If future fertility is not a concern, the decision to undergo a hysterectomy should be personalized, considering comorbidities and surgical risks (e.g., wound infection, nerve damage, subsequent adhesive disease, significant bleeding, death).
The 2008 American College of Obstetricians and Gynecologists guidelines address alternatives to hysterectomy for fibroids and emphasize individualizing treatment based on patient goals.4 Asymptomatic fibroids do not require treatment and should be managed expectantly. Physicians should not assume that fibroids are the cause of infertility without additional testing. Although myomectomy has a low complication rate, it carries a risk of fibroid recurrence.4 The American College of Obstetricians and Gynecologists is reviewing this AHRQ review to determine whether updates need to be made to its current guidelines.5
Adverse effects of nonsurgical therapies vary. GnRH agonists are expensive and may cause bone loss. Selective progesterone receptor modulators can cause headaches and breast tenderness. UAE increases the risk of amenorrhea and pregnancy complications. Pregnancy complications observed in the studies included a higher miscarriage rate and placenta previa and accreta. However, these findings may have been the result of publication bias, and additional studies are needed.4 Compared with hysterectomy, UAE was associated with a higher rate of readmissions and unscheduled visits.6
Editor's Note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence (SOE) ratings.
The views expressed in this article are those of the authors and do not reflect the policy or position of the U.S. Army Medical Department, Department of the Army, Department of Defense, or the U.S. government.
