
Am Fam Physician. 2019;99(8):505-514
Author disclosure: No relevant financial affiliations.
Palpable breast masses, mastalgia, and nipple discharge are commonly encountered symptoms in outpatient practice, causing significant patient anxiety and precipitating medical consultation. The initial workup includes a detailed clinical history and physical examination. Women presenting with a breast mass will require imaging and further assessment to exclude cancer. Diagnostic mammography is usually preferred, but ultrasonography is more sensitive in women younger than 30 years. Any suspicious mass detected on physical examination, mammography, or ultrasonography should undergo biopsy. In most cases, a core needle biopsy should be performed with imaging guidance for evaluation of a suspicious mass. Mastalgia is usually not an indication of underlying malignancy. Oral contraceptives, hormone therapy, some psychotropic drugs, and some cardiovascular agents have been associated with mastalgia. Focal breast pain should be evaluated with diagnostic imaging. Targeted ultrasonography localized to discrete areas of the breast can be used alone to evaluate focal breast pain in women younger than 30 years, and as an adjunct to mammography in women 30 years and older. Topical nonsteroidal anti-inflammatory drugs, such as diclofenac, are a first-line treatment option. The first step in the diagnostic evaluation of patients with nipple discharge is classification of the discharge as pathologic or physiologic. Nipple discharge is classified as pathologic if it is spontaneous, bloody, unilateral, or associated with a breast mass. Patients with pathologic discharge should undergo diagnostic imaging. Galactorrhea is the most common cause of physiologic discharge not associated with pregnancy or lactation. It occurs as a result of an endocrinopathy (hyperprolactinemia or thyroid dysfunction) or from the use of dopamine-inhibiting medications.
Common breast problems include breast mass, pain, and nipple discharge. Breast symptoms were reported in about 3% of all visits by female patients to family physicians.1 Over a 10-year period, 16% of women 40 to 69 years of age had breast problems, and 10% reported breast symptoms at the time of mammography.2,3 The prevalence of cancer among women who report breast symptoms is estimated to be less than 10%, and those with breast lumps have a higher risk of malignancy than those with breast pain.1–3 Although most breast symptoms have benign causes, symptoms can cause significant anxiety. Breast cancer is the most common cause of noncutaneous cancer and the second most common cause of death from cancer in the United States.4 Assessment and workup of breast symptoms are distinct from and do not supplant recommendations for breast cancer screening.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Ultrasonography is the preferred imaging modality in women younger than 30 years with a palpable breast mass. | C | 6–9 |
| Core needle biopsy with imaging guidance is the preferred method of tissue sampling for suspicious palpable breast masses. | C | 6–9 |
| Ultrasonography is the preferred imaging modality in women younger than 30 years with noncyclic, focal mastalgia and no palpable mass. | C | 12, 22, 23 |
| Diagnostic mammography should be performed in all women 30 years and older who have noncyclic, focal mastalgia and no palpable mass, and in all women 40 years and older who have noncyclic, nonfocal mastalgia and no palpable mass. | C | 12, 22, 23 |
| Diagnostic imaging is not needed in patients with cyclic mastalgia if routine screening mammography is up to date and physical examination findings are normal. | C | 7, 12, 24 |
| Topical nonsteroidal anti-inflammatory drugs such as diclofenac are first-line treatments for mastalgia. | B | 13, 14 |
| Diagnostic imaging is not needed in patients with physiologic nipple discharge if routine screening mammography is up to date and physical examination findings are normal. | C | 7, 9, 30 |
| Radiologic investigation should be performed in patients with nipple discharge that is spontaneous, unilateral, clear, serous, bloody, or associated with a mass. | C | 7, 30 |
Breast Mass
A thorough CBE in a symptomatic patient can guide the clinician's level of concern and help determine the next step in management. It may also detect lesions not found on imaging.6–8 CBE should start with visual inspection while the patient is seated with her hands on her hips. Any signs of nipple discharge, asymmetry, skin retraction, bulging, edema, erythema, or skin thickening should raise concern for malignancy. Inspection should be followed with palpation of the axillary, supraclavicular, and cervical lymph nodes. Finally, palpation of the breasts should be performed with the patient in the supine position.6 The duration of the CBE is the only variable that correlates with accuracy, and a thorough examination is recommended.2,9
Although CBE has poor predictive value for determining whether a breast mass is cancerous, some features of breast masses may help distinguish between benign and malignant lesions. Benign masses are more likely to be smaller, mobile, smooth, and regular. Malignant masses are typically larger, fixed, hard, and heterogeneous in texture.6 Documentation of a mass found on CBE should include size, consistency, distance from the areolar edge, and circumferential position on the breast.6 The sensitivity of CBE ranges from 49% to 69%, and specificity is 86% to 99%.2,9 Detection of malignancy increases when imaging is added to the evaluation.
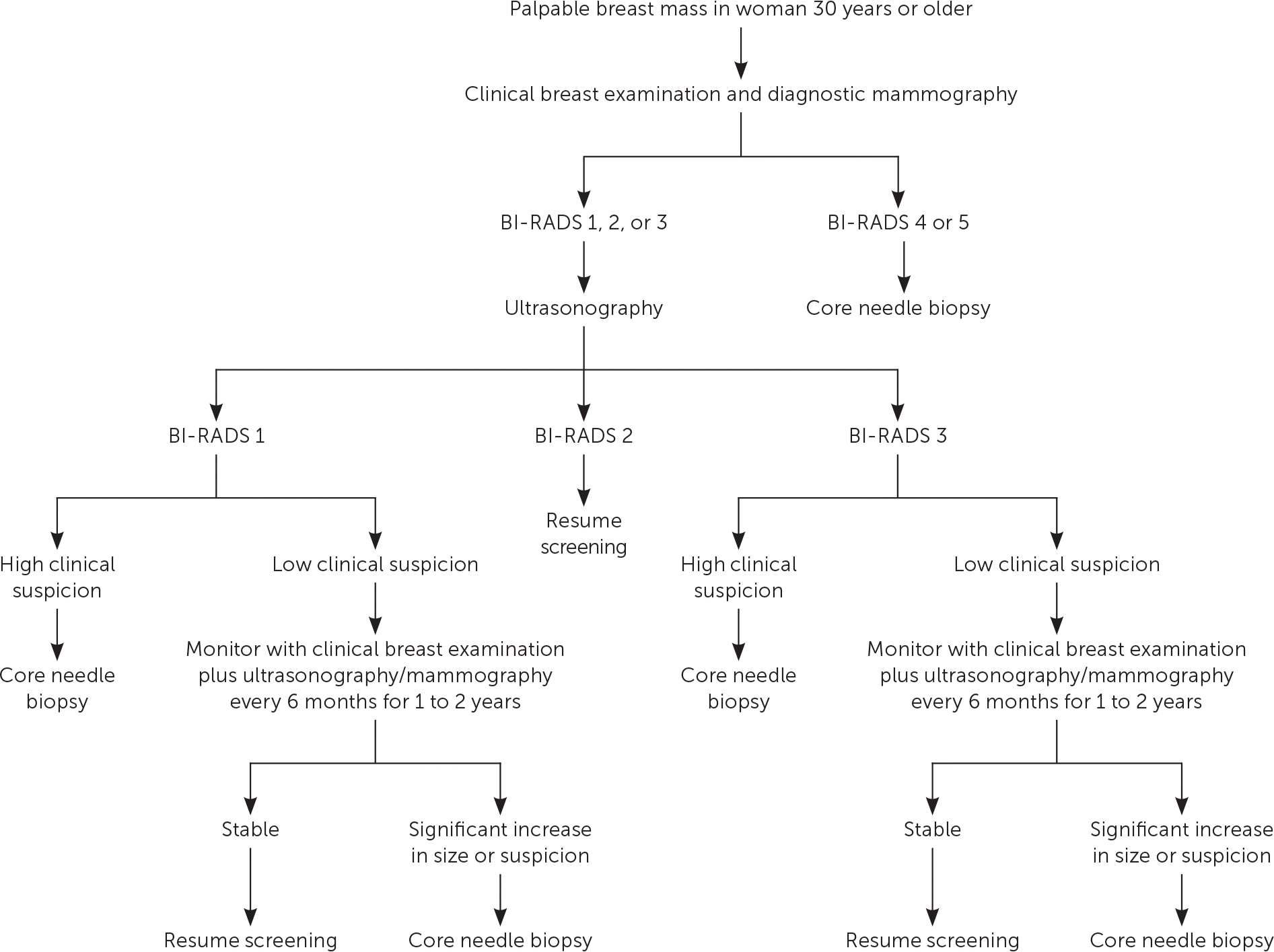
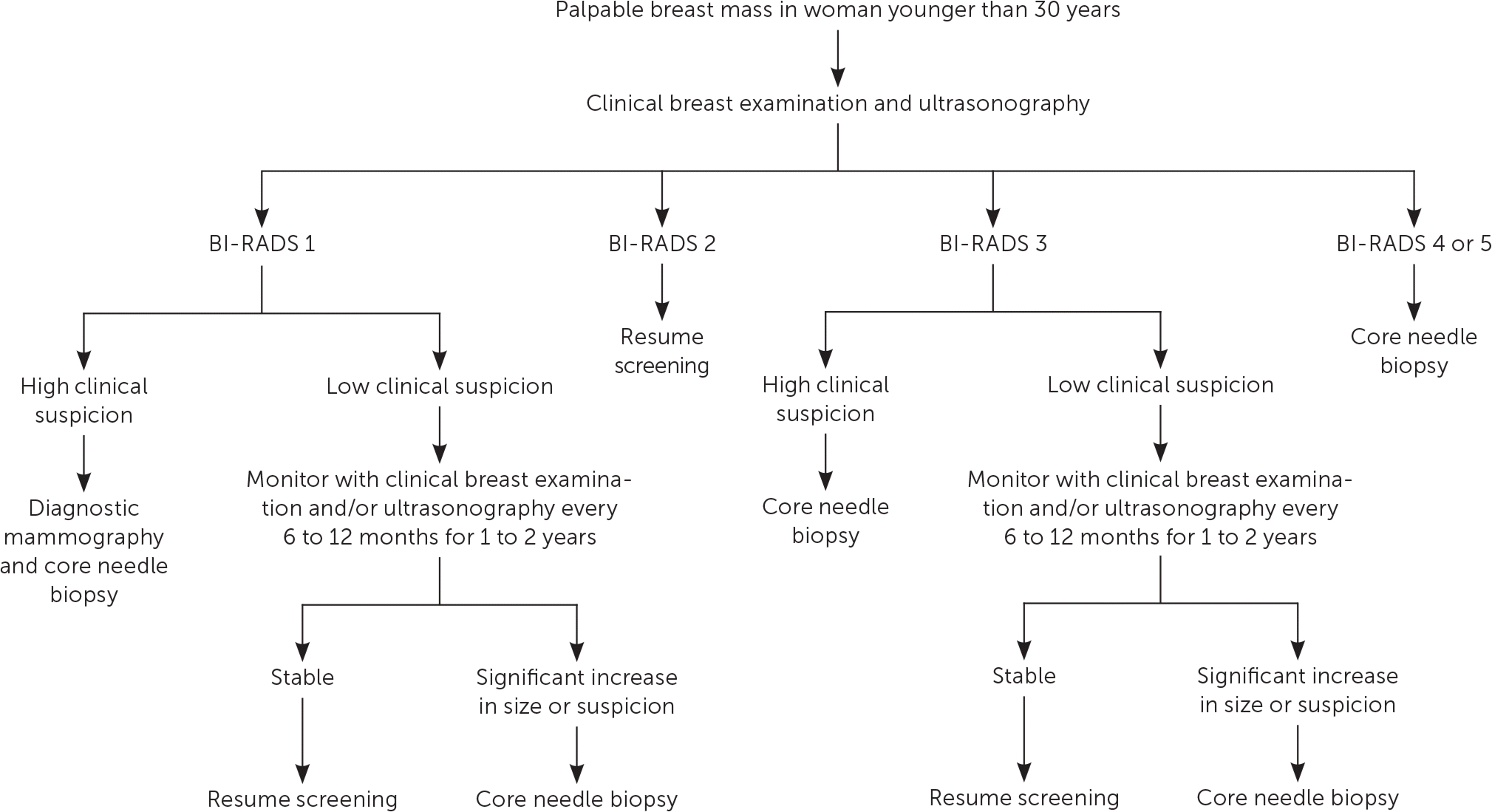
Figure 1 and Figure 2 outline the evaluation of palpable breast masses in women.7–9 Diagnostic mammography is the most appropriate initial imaging modality for women 40 years and older who present with a breast mass, whereas ultrasonography is recommended for women younger than 30 years.6–9 There is no clear evidence to support one imaging modality over the other in women 30 to 39 years of age, although many guidelines recommend evaluating these patients according to algorithms for women older than 40 years.6–9 Clinical imaging serves three purposes: to evaluate the mass, look for additional abnormalities in the breast, and guide biopsy.7–9 The clinician should ensure that imaging findings are concordant with the area of concern. The diagnostic accuracy for mammography is as high as 78%.10 Ultrasonography is generally performed at the same visit as mammography if there are any areas of concern. The U.S. Food and Drug Administration requires that all mammography reports be accompanied by a Breast Imaging Reporting and Data System (BI-RADS) categorization to direct management (Table 1).8,9
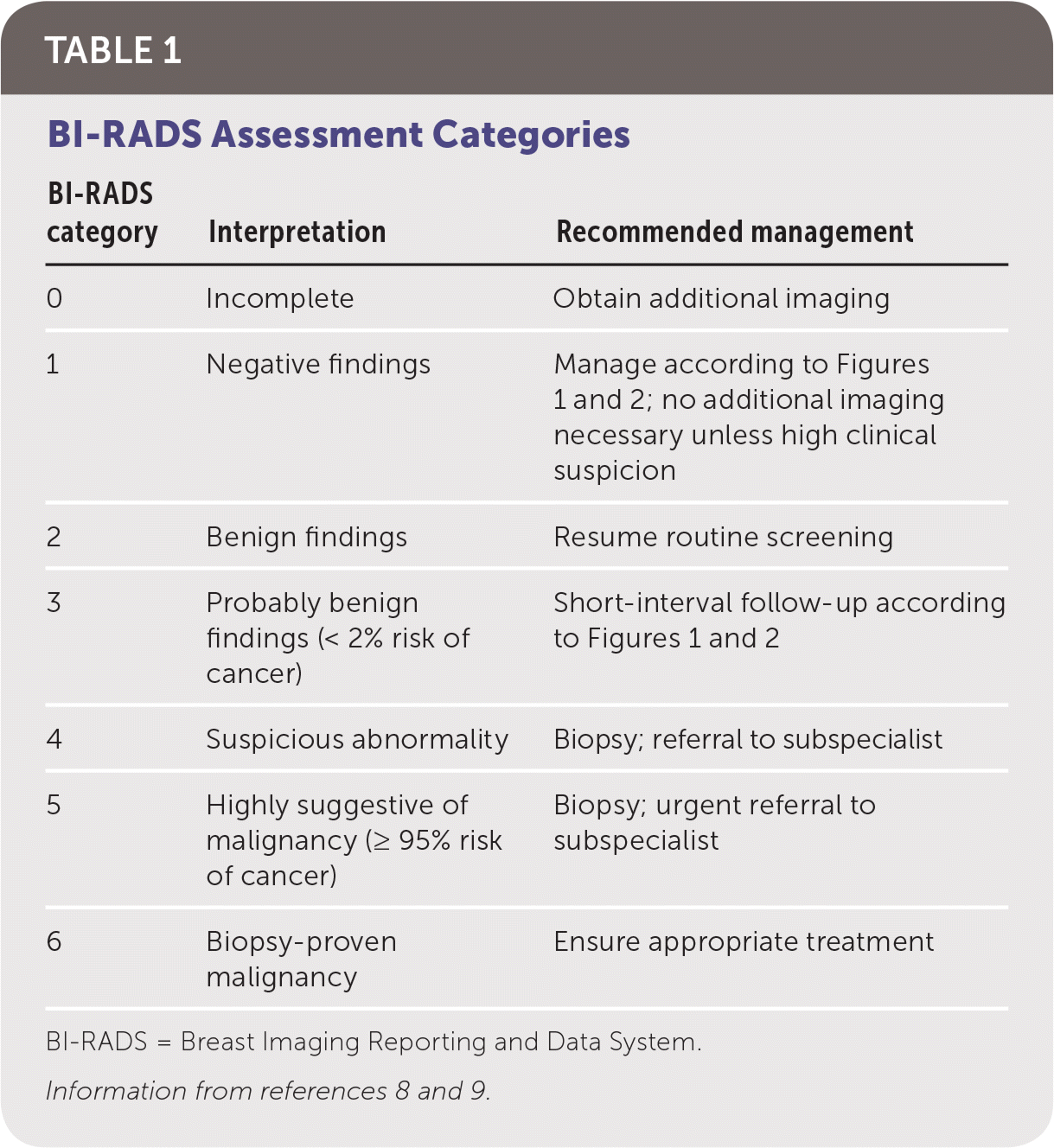
| BI-RADS category | Interpretation | Recommended management |
|---|---|---|
| 0 | Incomplete | Obtain additional imaging |
| 1 | Negative findings | Manage according to Figure 1 and Figure 2; no additional imaging necessary unless high clinical suspicion |
| 2 | Benign findings | Resume routine screening |
| 3 | Probably benign findings (< 2% risk of cancer) | Short-interval follow-up according to Figure 1 and Figure 2 |
| 4 | Suspicious abnormality | Biopsy; referral to subspecialist |
| 5 | Highly suggestive of malignancy (≥ 95% risk of cancer) | Biopsy; urgent referral to dsubspecialist |
| 6 | Biopsy-proven malignancy | Ensure appropriate treatment |
Magnetic resonance imaging is not recommended for evaluation of a palpable mass in most women.8 Although it is more sensitive than mammography, it is less specific and more expensive, and it has a high false-positive rate.8,9,11 Magnetic resonance imaging may be indicated in women who have undergone lumpectomy to determine whether a palpable mass is a recurrence or scarring from the procedure.8 It may also be indicated for screening in women older than 25 years who have a greater than 20% lifetime risk of breast cancer or who have received thoracic radiation therapy between 10 and 30 years of age.7 Risk assessment tools such as the National Cancer Institute's Breast Cancer Risk Assessment Tool are available to assess lifetime risk (https://www.cancer.gov/bcrisktool).
Because of the imperfect sensitivity and specificity of CBE and imaging, patients should be referred for tissue sampling if suspicious findings are noted at any stage of the evaluation, regardless of benign findings at other stages. A highly suspicious breast mass found on CBE should be biopsied regardless of imaging findings, and suspicious masses on imaging should be biopsied even if the CBE suggested benign findings.7–9
Imaging should be performed before biopsy because postbiopsy changes in the breast tissue may distort imaging findings.8 In most cases, a core needle biopsy should be performed for evaluation of a suspicious mass. Compared with fine-needle aspiration, core needle biopsy has superior sensitivity, specificity, and ability to detect possible malignant invasion.12 It also has a lower risk of scarring and complications, lower cost, similar accuracy, and faster recovery time than open biopsy. The accuracy of core needle biopsy improves with imaging guidance.6,8 A punch biopsy may be appropriate to evaluate skin abnormalities when no subcutaneous lesion is present. Fine-needle aspiration may be appropriate if ultrasonography identifies a simple cystic lesion. Complicated (BI-RADS 3) cysts may be followed with imaging surveillance or aspiration, whereas complex (BI-RADS 4) cysts should undergo biopsy because of their greater probability of malignancy.9
Mastalgia
Mastalgia is the second most common breast symptom leading to medical evaluation in the primary care setting.13,14 Although pain is often mild, up to 11% of women experience severe pain, and more than one-third of these patients report adverse effects on sleep and sexual activity.15 Cyclic mastalgia accounts for two-thirds of all breast pain cases and is most common in women in their 20s and 30s.16 Cyclic pain tends to be diffuse and bilateral, and often radiates to the axilla.15 It is thought to be induced by increased sensitivity of the breast parenchyma to hormonal stimulation during the luteal phase of the menstrual cycle.12 Noncyclic mastalgia has no temporal association with menses and may be focal or diffuse. Patients are usually older, often presenting in their 30s or 40s. Symptoms resolve spontaneously in nearly one-half of affected women.12,13 Noncyclic pain is thought to be inflammatory and has been associated with medication use (oral contraceptives, hormone therapy, some psychotropic agents, and some cardiovascular agents), breast trauma, infection, benign tumors, and ligamentous pain from pendulous breasts.12,16–19 Pain may also be referred from extramammary cardiopulmonary or gastrointestinal sources or inflammatory musculoskeletal conditions.16,20
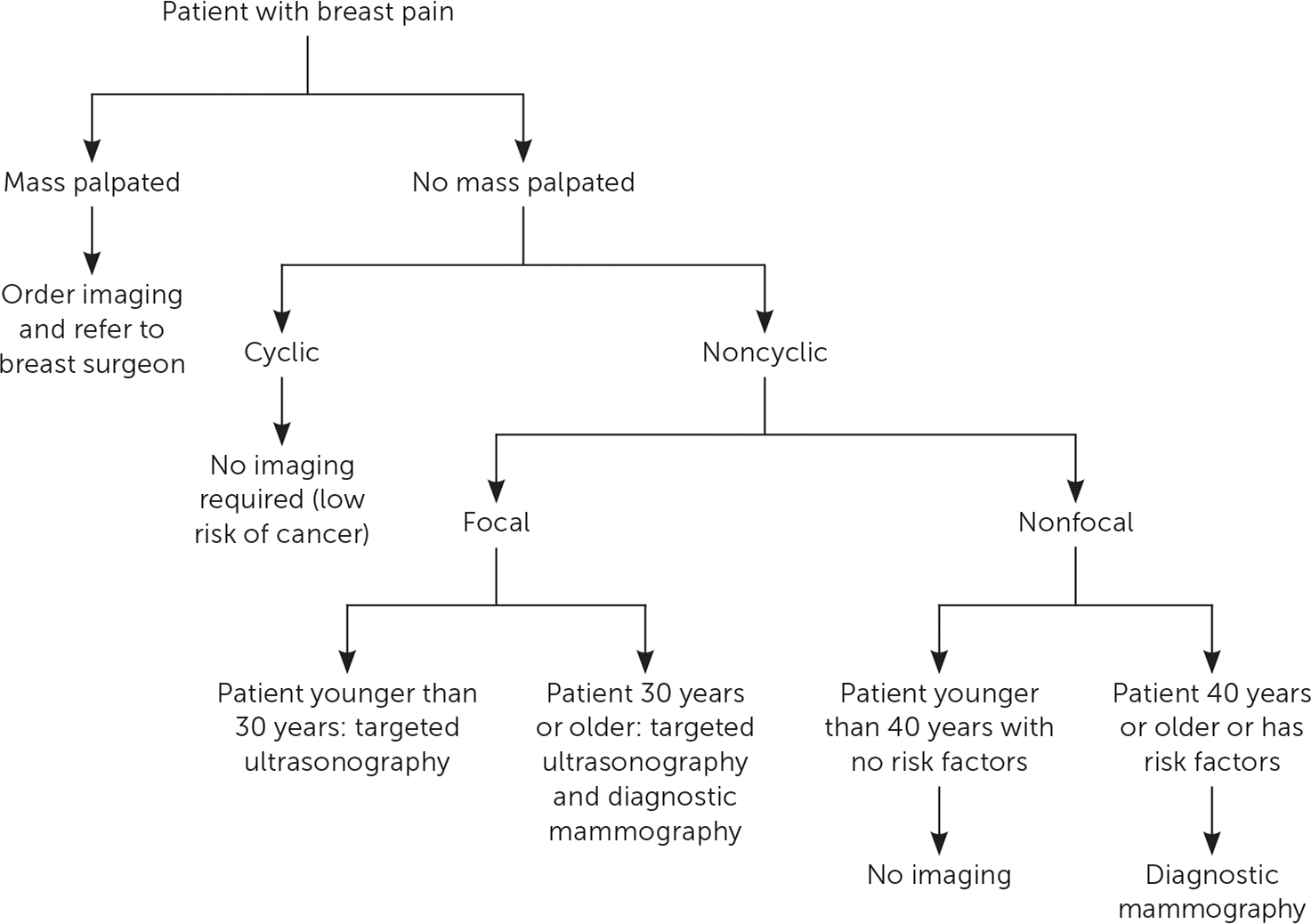
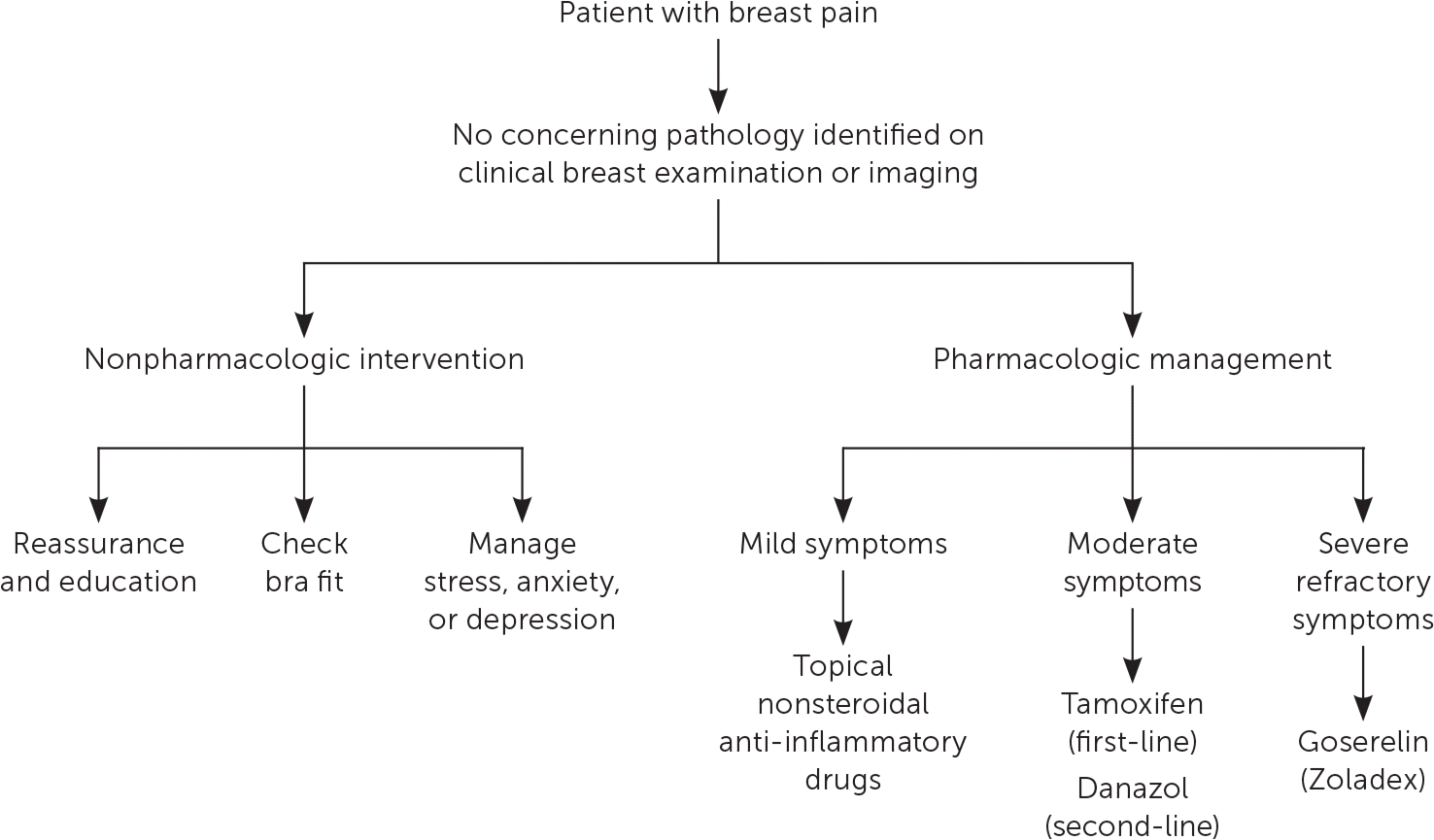
Evaluation of breast pain begins with a detailed history: pain profile (location, quality, severity, laterality, and temporal relation to menses), medication history, musculoskeletal triggers, impact on daily function, and family history of breast cancer.21 A CBE should follow. A palpable breast mass requires imaging and biopsy.8 In women with breast pain and no identifiable mass, the clinician may recommend imaging based on mastalgia characterization and patient age8,12,22–24 (Figure 312,22,23). Diagnostic imaging is not needed in patients with cyclic mastalgia if routine screening mammography is up to date and physical examination findings are normal.7,8,12,24 Noncyclic/focal mastalgia should trigger diagnostic imaging because of its rare but occasional association with underlying malignancy.8 Imaging may also identify a benign, treatable cause of noncyclic/focal pain.12,24 The risk of malignancy in patients with breast pain after normal CBE and mammography findings is approximately 0.5%.8,12
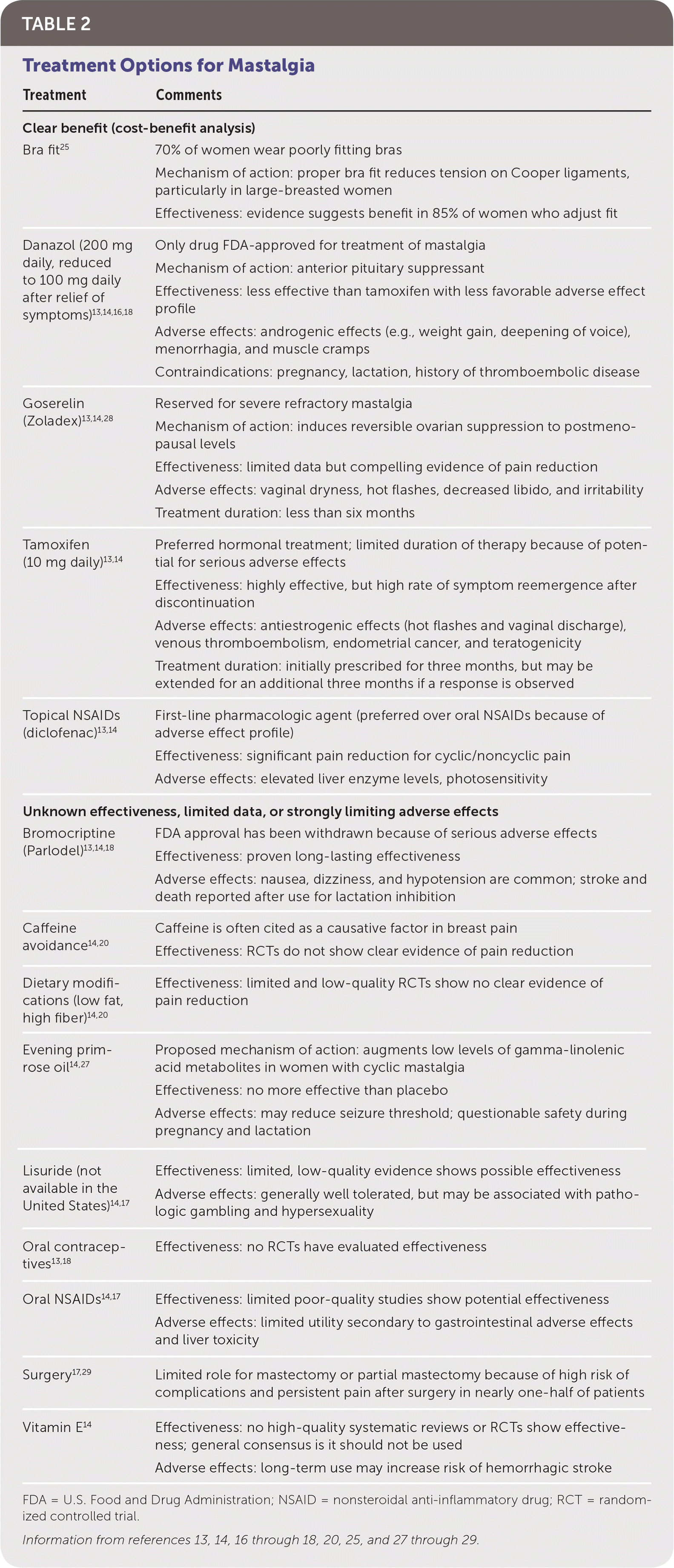
Although spontaneous resolution of breast pain is common, mastalgia can be chronic, necessitating a stepwise approach to management13,14,22 (Figure 413–15,23,25,26). Reassuring women with cyclic mastalgia that they are at low risk of breast cancer and recommending select lifestyle modifications have been shown to reduce symptoms.10,16,17,25 Topical nonsteroidal anti-inflammatory drugs such as diclofenac are the first-line pharmacologic treatment for cyclic and noncyclic mastalgia.13,14 Natural remedies such as vitamin E and evening primrose oil are commonly used despite limited data supporting their effectiveness.14,18,26 Caffeine intake, iodine deficiency, and dietary fat intake have not been definitively established as causal factors in mastalgia, and dietary modifications are not effective treatments.16,19,27 In instances of severe and refractory pain, clinicians may consider hormonal agents such as tamoxifen or danazol. Although several selective estrogen receptor modulators have been reviewed in the literature, tamoxifen is the most extensively studied and has clear benefit in up to 90% of women.14,18 Tamoxifen is more effective than danazol, with longer-lasting results and more tolerable adverse effects.13,14 Because of the significant adverse effects associated with these drugs, referral to a subspecialist should be considered before prescribing these agents. Table 2 provides a summary of various therapeutic options.13,14,16–18,20,25,27–29
| Treatment | Comments |
|---|---|
| Clear benefit (cost-benefit analysis) | |
| Bra fit25 | 70% of women wear poorly fitting bras Mechanism of action: proper bra fit reduces tension on Cooper ligaments, particularly in large-breasted women Effectiveness: evidence suggests benefit in 85% of women who adjust fit |
| Danazol (200 mg daily, reduced to 100 mg daily after relief of symptoms)13,14,16,18 | Only drug FDA-approved for treatment of mastalgia Mechanism of action: anterior pituitary suppressant Effectiveness: less effective than tamoxifen with less favorable adverse effect profile Adverse effects: androgenic effects (e.g., weight gain, deepening of voice), menorrhagia, and muscle cramps Contraindications: pregnancy, lactation, history of thromboembolic disease |
| Goserelin (Zoladex)13,14,28 | Reserved for severe refractory mastalgia Mechanism of action: induces reversible ovarian suppression to postmenopausal levels Effectiveness: limited data but compelling evidence of pain reduction Adverse effects: vaginal dryness, hot flashes, decreased libido, and irritability Treatment duration: less than six months |
| Tamoxifen (10 mg daily)13,14 | Preferred hormonal treatment; limited duration of therapy because of potential for serious adverse effects Effectiveness: highly effective, but high rate of symptom reemergence after discontinuation Adverse effects: antiestrogenic effects (hot flashes and vaginal discharge), venous thromboembolism, endometrial cancer, and teratogenicity Treatment duration: initially prescribed for three months, but may be extended for an additional three months if a response is observed |
| Topical NSAIDs (diclofenac)13,14 | First-line pharmacologic agent (preferred over oral NSAIDs because of adverse effect profile) Effectiveness: significant pain reduction for cyclic/noncyclic pain Adverse effects: elevated liver enzyme levels, photosensitivity |
| Unknown effectiveness, limited data, or strongly limiting adverse effects | |
| Bromocriptine (Parlodel)13,14,18 | FDA approval has been withdrawn because of serious adverse effects Effectiveness: proven long-lasting effectiveness Adverse effects: nausea, dizziness, and hypotension are common; stroke and death reported after use for lactation inhibition |
| Caffeine avoidance14,20 | Caffeine is often cited as a causative factor in breast pain Effectiveness: RCTs do not show clear evidence of pain reduction |
| Dietary modifications (low fat, high fiber)14,20 | Effectiveness: limited and low-quality RCTs show no clear evidence of pain reduction |
| Evening primrose oil14,27 | Proposed mechanism of action: augments low levels of gamma-linolenic acid metabolites in women with cyclic mastalgia Effectiveness: no more effective than placebo Adverse effects: may reduce seizure threshold; questionable safety during pregnancy and lactation |
| Lisuride (not available in the United States)14,17 | Effectiveness: limited, low-quality evidence shows possible effectiveness Adverse effects: generally well tolerated, but may be associated with pathologic gambling and hypersexuality |
| Oral contraceptives13,18 | Effectiveness: no RCTs have evaluated effectiveness |
| Oral NSAIDs14,17 | Effectiveness: limited poor-quality studies show potential effectiveness Adverse effects: limited utility secondary to gastrointestinal adverse effects and liver toxicity |
| Surgery17,29 | Limited role for mastectomy or partial mastectomy because of high risk of complications and persistent pain after surgery in nearly one-half of patients |
| Vitamin E14 | Effectiveness: no high-quality systematic reviews or RCTs show effectiveness; general consensus is it should not be used Adverse effects: long-term use may increase risk of hemorrhagic stroke |
Nipple Discharge
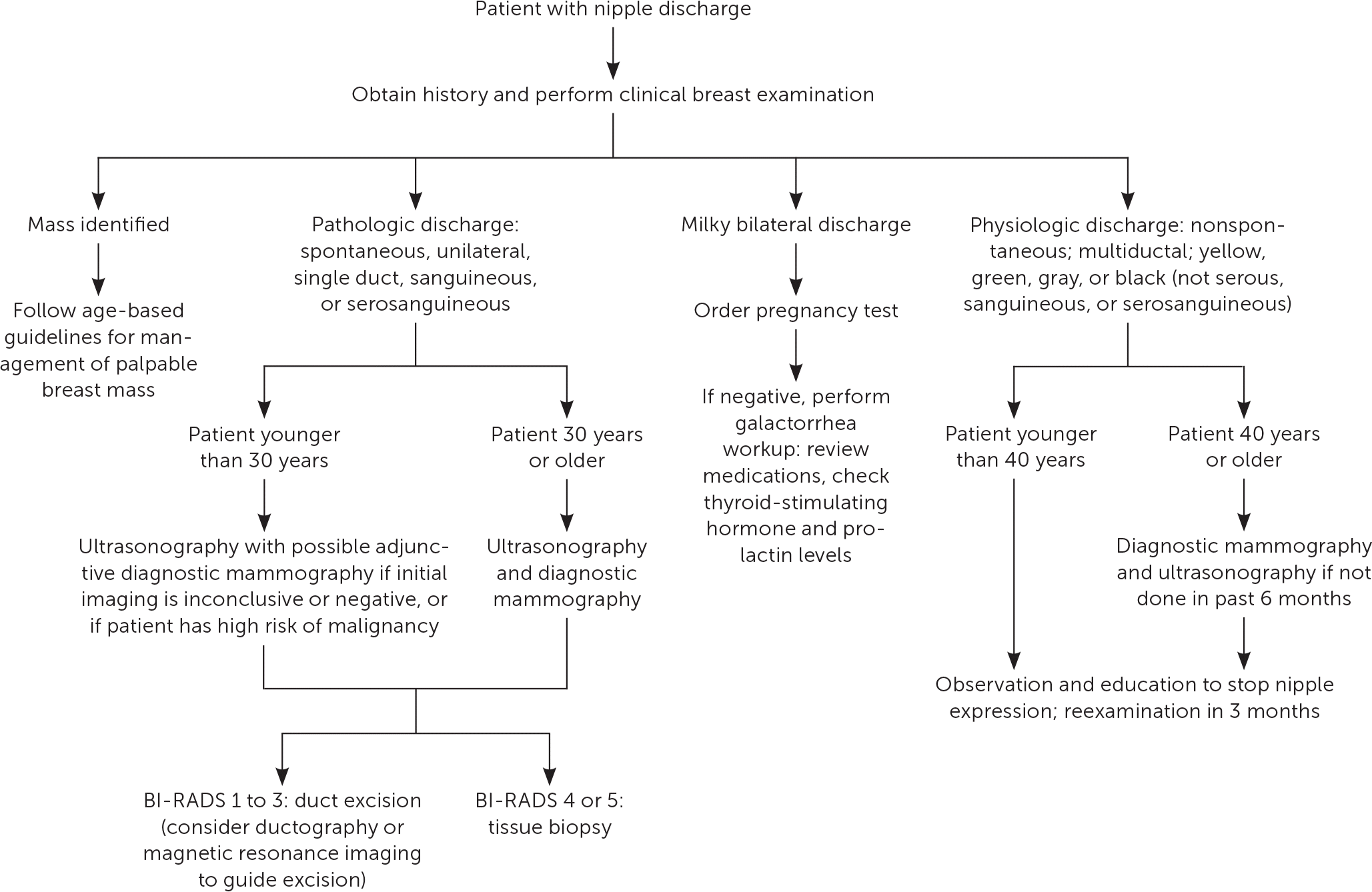
Nipple discharge is a common symptom among women of reproductive age, with most women experiencing at least one episode.30 Although nipple discharge is predominantly physiologic or due to a benign etiology, an underlying malignancy is identified in up to 21% of patients with pathologic discharge who undergo biopsy.30 The initial workup includes a comprehensive history and physical examination, with the primary aim of distinguishing between normal lactation, nonpuerperal galactorrhea, and pathologic discharge. Figure 5 outlines the workup for nipple discharge.18,30,31
PHYSIOLOGIC DISCHARGE
Physiologic discharge is generally bilateral, multiductal, negative for blood (regardless of color), and associated with nipple stimulation or breast compression.32 Most women can express fluid from their breast during their reproductive years. During pregnancy and lactation, the mammary glands excrete milk or colostrum in response to estrogen, progesterone, prolactin, and oxytocin. This physiologic discharge may persist for up to one year after pregnancy and cessation of breast-feeding.18 The presence of spontaneous bilateral lactation outside of pregnancy and the postpartum period is termed galactorrhea. Galactorrhea does not represent intrinsic breast disease and is commonly caused by hyperprolactinemia.18 Elevated prolactin levels may be linked to hypothalamic or pituitary lesions (tumors or infiltrative disorders), systemic disease (hypothyroidism or renal insufficiency), or dopamine-inhibiting medications.18,31,32 All patients with true galactorrhea should have a human chorionic gonadotropin pregnancy test to rule out pregnancy, followed by measurement of prolactin and thyroid-stimulating hormone levels.32 A comprehensive medication review should be performed to rule out iatrogenic causes (eTable A). If the history, physical examination findings, and routine screening mammography are consistent with physiologic discharge, no additional imaging is indicated.30,33 Avoidance of nipple expression may expedite resolution.18

| Drug class | Agent |
|---|---|
| Antihypertensive agents | MethyldopaA1 ReserpineA1 VerapamilA1,A2 |
| Gastrointestinal agents | Cimetidine (Tagamet) Metoclopramide (Reglan)A2,A3 |
| Hormones | EstrogenA1,A3 Oral contraceptivesA1–A3 |
| Opioids | CodeineA1 HeroinA1 MethadoneA3 Morphine |
| Psychotropic agents | AntipsychoticsA1 Monoamine oxidase inhibitorsA3 NeurolepticsA4 Selective serotonin reuptake inhibitorsA1 Tricyclic antidepressantsA1,A3 |
PATHOLOGIC DISCHARGE
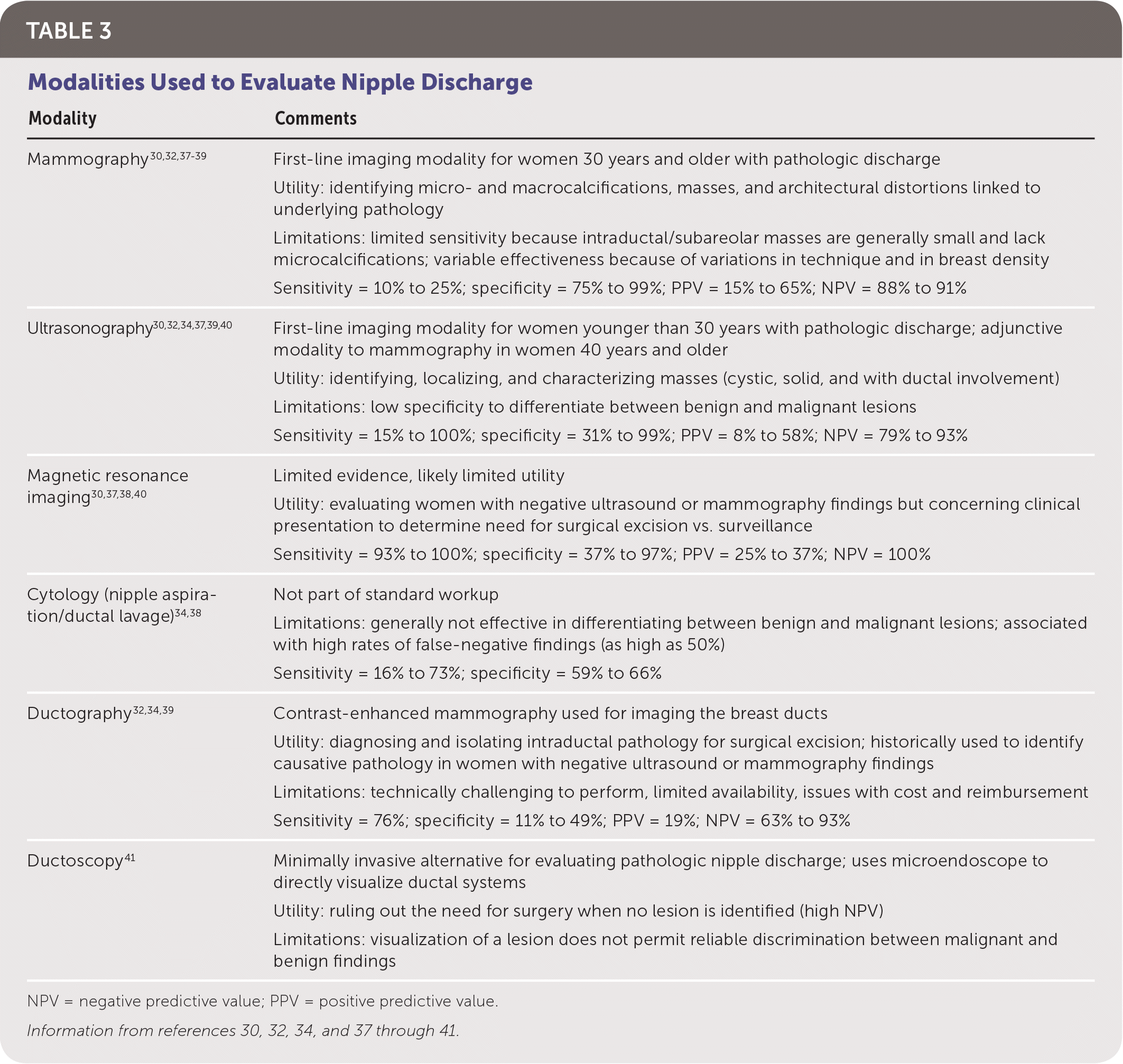
Pathologic discharge is generally spontaneous and unilateral, and originates from a single duct opening on a nipple. It may be bloody, serous, serosanguineous, or watery.30 The differential diagnosis includes intraductal papilloma, ductal ectasia, and breast carcinoma.30 If discharge is deemed pathologic, age-appropriate diagnostic imaging with mammography and/or ultrasonography is indicated.30 Studies have shown a low risk of malignancy when diagnostic studies are negative. The clinical utility of cytology is limited because of its high rate of false-negative findings.34 Imaging results of BI-RADS 4 or 5 require tissue biopsy. For imaging results of BI-RADS 1 to 3, management options include duct excision or follow-up with physical examination after six months and repeat diagnostic imaging for one to two years or until discharge resolves.7,35 Duct excision, potentially localized by ultrasonography, magnetic resonance imaging, or ductography, is preferred to rule out malignancy.30,36,37 Duct excision may provide the additional benefit of symptomatic relief.35 Table 3 outlines imaging modalities and indications.30,32,34,37–41
| Modality | Comments |
|---|---|
| Mammography30,32,37–39 | First-line imaging modality for women 30 years and older with pathologic discharge Utility: identifying micro- and macrocalcifications, masses, and architectural distortions linked to underlying pathology Limitations: limited sensitivity because intraductal/subareolar masses are generally small and lack microcalcifications; variable effectiveness because of variations in technique and in breast density Sensitivity = 10% to 25%; specificity = 75% to 99%; PPV = 15% to 65%; NPV = 88% to 91% |
| Ultrasonography30,32,34,37,39,40 | First-line imaging modality for women younger than 30 years with pathologic discharge; adjunctive modality to mammography in women 40 years and older Utility: identifying, localizing, and characterizing masses (cystic, solid, and with ductal involvement) Limitations: low specificity to differentiate between benign and malignant lesions Sensitivity = 15% to 100%; specificity = 31% to 99%; PPV = 8% to 58%; NPV = 79% to 93% |
| Magnetic resonance imaging30,37,38,40 | Limited evidence, likely limited utility Utility: evaluating women with negative ultrasound or mammography findings but concerning clinical presentation to determine need for surgical excision vs. surveillance Sensitivity = 93% to 100%; specificity = 37% to 97%; PPV = 25% to 37%; NPV = 100% |
| Cytology (nipple aspiration/ductal lavage)34,38 | Not part of standard workup Limitations: generally not effective in differentiating between benign and malignant lesions; associated with high rates of false-negative findings (as high as 50%) Sensitivity = 16% to 73%; specificity = 59% to 66% |
| Ductography32,34,39 | Contrast-enhanced mammography used for imaging the breast ducts Utility: diagnosing and isolating intraductal pathology for surgical excision; historically used to identify causative pathology in women with negative ultrasound or mammography findings Limitations: technically challenging to perform, limited availability, issues with cost and reimbursement Sensitivity = 76%; specificity = 11% to 49%; PPV = 19%; NPV = 63% to 93% |
| Ductoscopy41 | Minimally invasive alternative for evaluating pathologic nipple discharge; uses microendoscope to directly visualize ductal systems Utility: ruling out the need for surgery when no lesion is identified (high NPV) Limitations: visualization of a lesion does not permit reliable discrimination between malignant and benign findings |
This article updates previous articles on this topic by Salzman, et al.,42 and by Morrow.43
Data Sources: A PubMed search was completed in Clinical Queries using the key terms breast symptoms, breast lump, breast pain, mastalgia, and nipple discharge. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Agency for Healthcare Research and Quality evidence reports, the U.S. Preventive Services Task Force, the Centers for Disease Control and Prevention, the Cochrane database, Essential Evidence Plus, the Institute for Clinical Systems Improvement, and the National Guideline Clearinghouse database. Search dates: January to November 2018.
