Am Fam Physician. 2019;99(9):online
Author disclosure: No relevant financial affiliations.

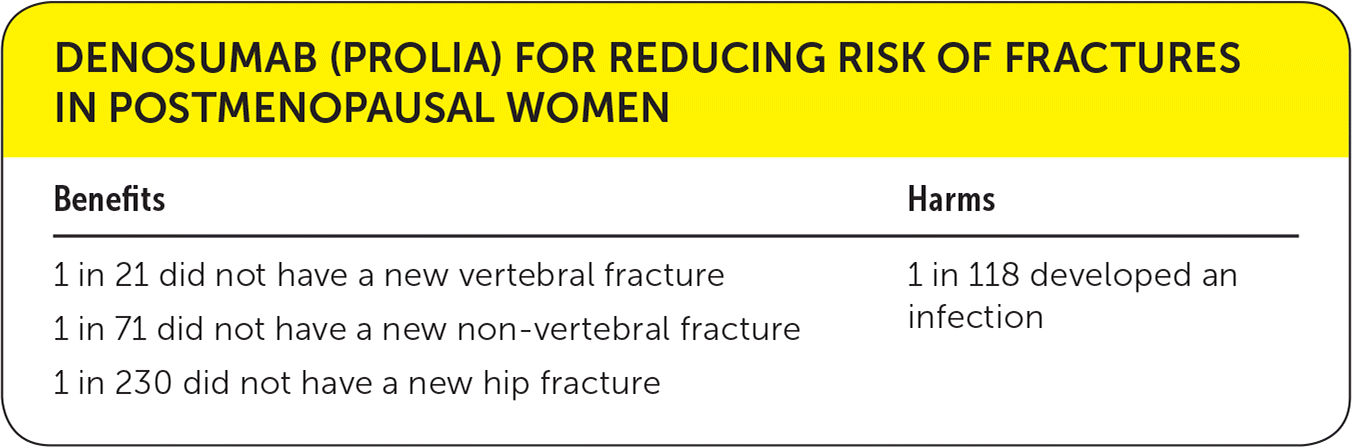
| Benefits | Harms |
|---|---|
| 1 in 21 did not have a new vertebral fracture | 1 in 118 developed an infection |
| 1 in 71 did not have a new non-vertebral fracture | |
| 1 in 230 did not have a new hip fracture |
Details for This Review
Study Population: Postmenopausal women with diagnosed osteoporosis
Efficacy End Points: Prevention of new vertebral and nonvertebral fractures
Harm End Points: Infection, neoplasm, death
Narrative: As bone density decreases, people are at an increased risk of fractures.1 Denosumab (Prolia) is a fully human monoclonal antibody that binds the receptor activator of nuclear factor kappa B ligand (RANKL), which prevents its interaction with the osteoclast and osteoclast precursor surface receptor, RANK. This inhibits osteoclast-mediated bone resorption by blocking osteoclast function, formation, and survival.2,3 Studies have demonstrated an increase in bone mineral density with the administration of denosumab in postmenopausal women.4,5 This review assesses whether the increase in bone mineral density translates into a reduction in the risk of osteoporosis-related fractures.
The FREEDOM trial is the largest randomized controlled trial to compare denosumab with placebo in the prevention of fractures in postmenopausal women with osteoporosis.2 Women between 60 and 90 years of age with a bone mineral density T-score of less than −2.5 (consistent with the typical definition of osteoporosis) at the lumbar spine or total hip were included in the trial. Patients were randomly assigned to receive subcutaneous injections of 60 mg of denosumab or placebo every six months for 36 months. In this trial, the primary end point was new vertebral fractures based on semi-quantitative grading scales of lateral spine radiographs.2 The treatment with denosumab was associated with significantly lower risk of new vertebral fractures (relative risk [RR] = 0.32;95% CI, 0.26 to 0.41; absolute risk difference [ARD] = 4.8%; number needed to treat [NNT] = 21). Secondary outcomes included nonvertebral fractures (NNT = 71), hip fractures (NNT = 230), new clinical vertebral fractures (NNT = 62), and multiple (at least two) new vertebral fractures (NNT = 103). The study found no significant difference in the incidence of infection, death, or neoplasm.2
A 2014 meta-analysis of 13 trials examined the safety of denosumab in 15,263 postmenopausal women with documented osteoporosis who were followed between nine months to three years after initiation of therapy.3 This meta-analysis found a nonsignificant reduction in the risk of nonvertebral fractures with the administration of denosumab (RR = 0.86; 95% CI, 0.74 to 1.00). The meta-analysis found the difference in incidence of death or neoplasm to be nonsignificant. The difference in rate of infection was not statistically significant between the groups (RR = 1.23; 95% CI, 1.00 to 1.52).2 Using this information, we calculated the number needed to harm (NNH) because the sample size for the meta-analysis was larger than the FREEDOM trial2 and the objective of the meta-analysis specifically was to assess the safety of the treatment.3
Another 2014 systematic review compared the effectiveness of various pharmacologic treatments in reducing risk of fractures.6 This analysis confirmed the efficacy of denosumab in reducing the risk of fractures in postmenopausal women. For different pharmacologic treatments including various bisphosphonates, bis-phosphate derivatives, teriparatide (Forteo), raloxifene (Evista), or denosumab; the NNT for vertebral fractures was in the range of 60 to 89 and the NNT for nonvertebral fracture was 50 to 60.6 The review also found denosumab to have an NNH of 118 for infection (RR = 1.28; 95% CI, 1.02 to 1.60; ARD = 0.85%).6
Caveats: The meta-analysis by Zhou and colleagues3 included 11 randomized controlled trials, but the FREEDOM2 trial was the largest study with that sample size accounting for about 60% of the total number of participants in all studies. The existing evidence supports the safety of denosumab, but the follow-up period for the trials (ranging from nine months to three years) may have been too short for assessing the harm end points of neoplasm, death, or infection. More follow-up is needed to understand the long-term safety profile of this treatment.
One group of patients in the FREEDOM trial was followed for an additional seven years. In this follow-up study, the rates of serious adverse events for participants treated with denosumab remained low (11.5 and 14.4 per 100 participant-years).7 Denosumab is marketed under brand names Prolia and Xgeva. As of October 2018, the price of one 60-mg syringe or vial, which has to be administered every six months, is approximately $1,200 to $1,400 (estimated retail price based on information obtained at http://goodrx.com). The manufacturer’s website lists examples of possible infections associated with denosumab as infections of “skin, lower stomach, bladder, ear, or the inner layer of the heart (endocarditis)”.8
Denosumab appears to be effective in reducing the risk of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. Treatment with denosumab does not increase the risk of cancer or death but might increase the risk of infection. However, given the nonstatistically significant impact on nonvertebral fractures and the uncertainty of longer-term harms because of relatively short follow-up periods, we assigned this treatment a color recommendation of yellow (unclear benefit; more studies required).