Am Fam Physician. 2019;99(9):545-546
Author disclosure: No relevant financial affiliations.
Clinical Question
Compared with clomiphene (Clomid), are aromatase inhibitors such as letrozole (Femara) effective treatments for subfertile women with polycystic ovary syndrome (PCOS) who are trying to conceive?
Evidence-Based Answer
When treated with letrozole, subfertile women with PCOS who are trying to conceive have increased chances of pregnancy (number needed to treat [NNT] = 11) and live birth (NNT = 10) compared with those treated with clomiphene. The risk of adverse outcomes including miscarriage, ovarian hyperstimulation syndrome, and multiple pregnancy is not increased.1 (Strength of Recommendation: A, based on consistent, good-quality patient-oriented evidence.)
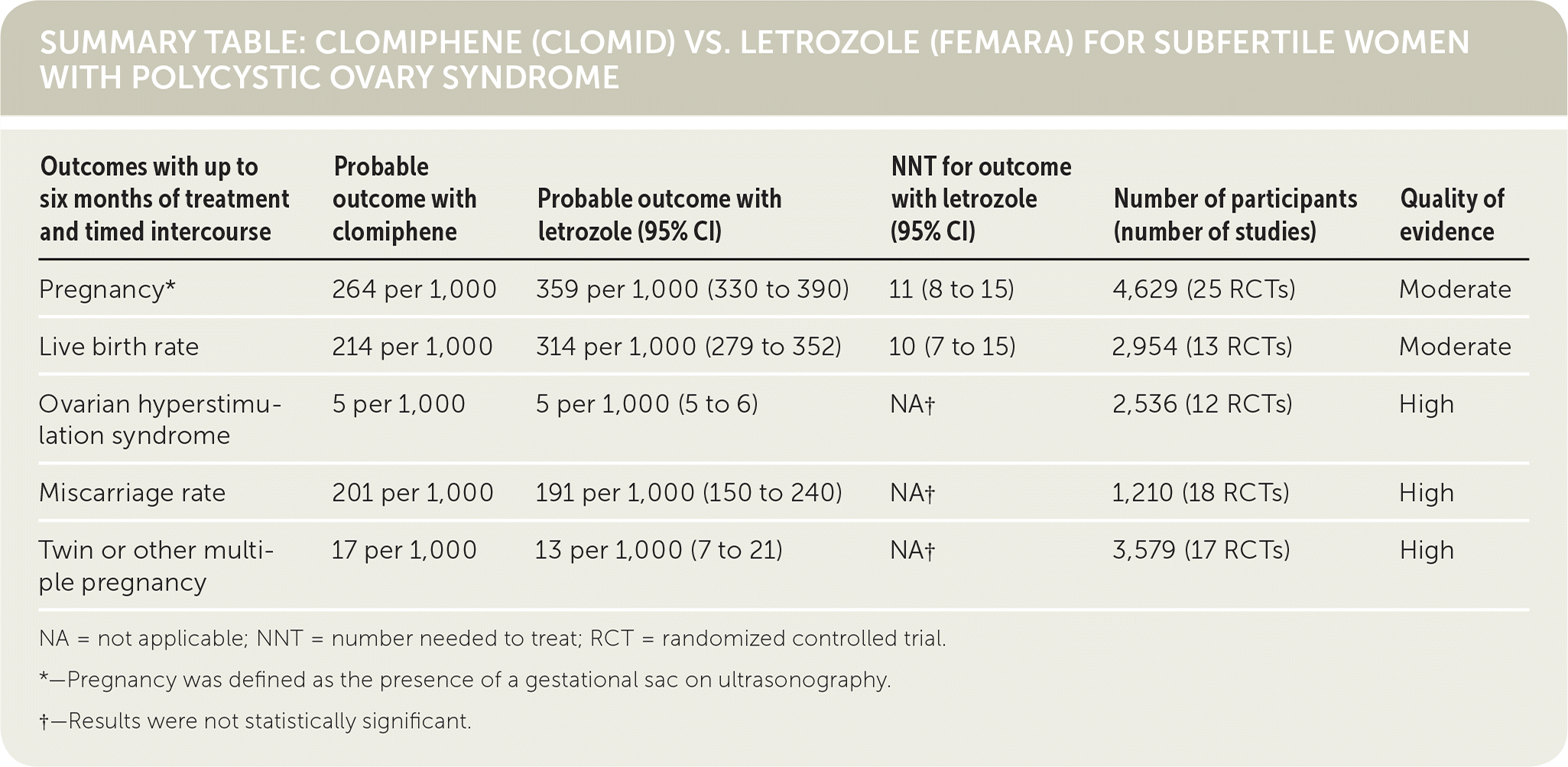
| Outcomes with up to six months of treatment and timed intercourse | Probable outcome with clomiphene | Probable outcome with letrozole (95% CI) | NNT for outcome with letrozole (95% CI) | Number of participants (number of studies) | Quality of evidence |
|---|---|---|---|---|---|
| Pregnancy* | 264 per 1,000 | 359 per 1,000 (330 to 390) | 11 (8 to 15) | 4,629 (25 RCTs) | Moderate |
| Live birth rate | 214 per 1,000 | 314 per 1,000 (279 to 352) | 10 (7 to 15) | 2,954 (13 RCTs) | Moderate |
| Ovarian hyperstimulation syndrome | 5 per 1,000 | 5 per 1,000 (5 to 6) | NA† | 2,536 (12 RCTs) | High |
| Miscarriage rate | 201 per 1,000 | 191 per 1,000 (150 to 240) | NA† | 1,210 (18 RCTs) | High |
| Twin or other multiple pregnancy | 17 per 1,000 | 13 per 1,000 (7 to 21) | NA† | 3,579 (17 RCTs) | High |
Practice Pointers
PCOS is the most common cause of oligomenorrhea and amenorrhea worldwide,1 affecting one in 10 U.S. women of childbearing age.2 Women with PCOS often experience anovulation. Clomiphene has been the most widely used treatment for infertility in this group. Both clomiphene and letrozole are given at the beginning of a menstrual cycle to improve the chances of ovulation and are followed by timed intercourse (or intrauterine insemination). The authors sought to determine if letrozole, an aromatase inhibitor, is as safe and effective as clomiphene for PCOS-associated infertility.
This Cochrane review included 42 randomized controlled trials (RCTs) comparing letrozole with clomiphene. The trials were from eight different countries and included a total of 7,935 women 18 to 40 years of age with anovulatory PCOS.1 Only one trial was performed in the United States. The primary analysis included studies of ovulation induction followed by timed intercourse. The quality of evidence was moderate for the primary outcome (live birth rate) and high for secondary outcomes (adverse effects).
In the analysis, women treated with letrozole vs. clomiphene had a higher incidence of live birth (treatment difference = 10% [95% CI, 6.5% to 13.8%]; NNT = 10 [95% CI, 7 to 15]). Clinical pregnancy (defined as the presence of a gestational sac on ultrasonography) was more common with letrozole (treatment difference = 9.5% [95% CI, 6.6% to 12.6%]; NNT = 11 [95% CI, 8 to 15]). The absolute risk of ovarian hyperstimulation syndrome, resulting in ovarian enlargement, ascites, and occasionally more serious complications,3 was low (0.5%) and similar in both groups. Miscarriage rates (approximately 20% in each group) and multiple pregnancy rates (approximately 1.5% in each group) were also not significantly different.
Canadian and U.S. obstetric society guidelines were updated in 2018 to list letrozole as first-line medical therapy for women with anovulatory PCOS who are trying to conceive.4,5 Women should be counseled that use for this indication is still considered off-label in both countries.4,5 Letrozole and clomiphene are typically administered for five days starting on day 3, 4, or 5 of a patient’s menstrual cycle.4 Letrozole may be more cost-effective ($11.73 for 30 tablets compared with $30.47 for 30 tablets of clomiphene).6
The practice recommendations in this activity are available at http://www.cochrane.org/CD010287.
Editor’s Note: The numbers needed to treat, confidence intervals, and treatment difference percentages reported in this Cochrane for Clinicians were calculated by the author based on raw data provided in the original Cochrane review.