Am Fam Physician. 2019;99(9):547-556
See related AFP Community Blog post: Introducing Dr. Mike Arnold, the first Jay Siwek Medical Editing Fellow
Author disclosure: No relevant financial affiliations.
Interventional radiology employs image-guided techniques to perform minimally invasive procedures for diagnosis and treatment. Interventional radiology is often used to place central venous catheters and subcutaneous ports, with some evidence of benefit over surgical placement. Arterial embolization procedures are used to manage many types of hemorrhage and are highly effective for severe postpartum hemorrhage. Vascular interventions, such as endovascular treatment of varicosities, acute limb ischemia, and pulmonary embolism, are superior to surgical interventions. For chronic limb ischemia and deep venous thrombosis, the choice of therapy is not as clear. Inferior vena cava filters can be placed and removed endovascularly, but there is a significant risk of complications that increases over time. Vascular interventions can be effective for scrotal varicocele and uterine fibroids, although fibroid treatment is limited by high recurrence rates. Image-guided percutaneous drainage and biopsy have become standard of care. Interventional approaches are being used in oncology for local diagnosis and treatment. Percutaneous ablation and targeted delivery of chemotherapy and radiation therapy are being developed as alternatives when surgery is not practical. Vertebroplasty and kyphoplasty provide significant pain and functional improvement in patients with spinal metastases.
Interventional radiology employs image-guided techniques to perform minimally invasive procedures, providing lower-risk alternatives to many traditional medical and surgical therapies. Since the advent of interventional radiology in the 1960s, its role has expanded to encompass the diagnosis and treatment of diseases across multiple body systems.1 The treatments discussed in this article represent a sample of what interventional radiology can offer to family physicians. Guidelines regarding procedural bleeding risks and recommended anticoagulation management are shown in Table 1.2,3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Overall complications and costs are reduced when ports are placed with interventional radiography rather than surgically. | B | 5–7 |
| Endovascular treatments of abdominal aortic aneurysms have improved 30-day mortality and equivalent total mortality compared with surgery. | A | 14 |
| Endovascular treatments for varicose veins have similar success rates and lower complication rates than surgery. | A | 17, 18 |
| Transarterial embolization for postpartum hemorrhage has a high success rate and can preserve fertility. | B | 24, 29 |
| Catheter-directed thrombolysis for massive and submassive pulmonary embolism has mortality benefits over anticoagulation alone. | B | 49–51 |
| Interventional percutaneous drainage and biopsy procedures have success rates that are at least equivalent to open surgical approaches. | B | 58–60, 64, 65, 67, 68 |
| Vertebroplasty and kyphoplasty provide significant pain and functional benefits to patients with painful spinal metastases. | B | 78 |
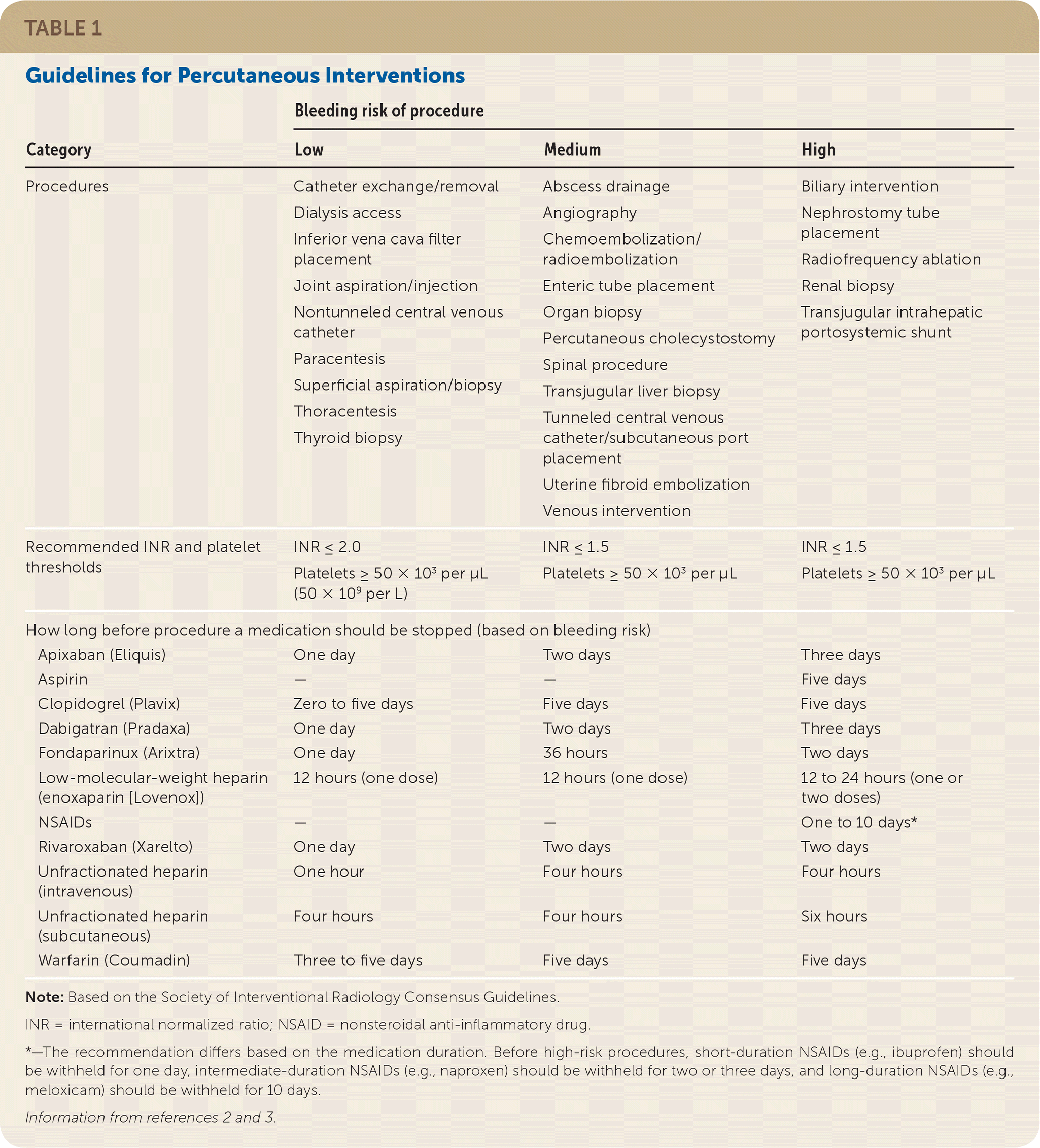
| Category | Bleeding risk of procedure | ||
|---|---|---|---|
| Low | Medium | High | |
| Procedures | Catheter exchange/removal Dialysis access Inferior vena cava filter placement Joint aspiration/injection Nontunneled central venous catheter Paracentesis Superficial aspiration/biopsy Thoracentesis Thyroid biopsy | Abscess drainage Angiography Chemoembolization/radioembolization Enteric tube placement Organ biopsy Percutaneous cholecystostomy Spinal procedure Transjugular liver biopsy Tunneled central venous catheter/subcutaneous port placement Uterine fibroid embolization Venous intervention | Biliary intervention Nephrostomy tube placement Radiofrequency ablation Renal biopsy Transjugular intrahepatic portosystemic shunt |
| Recommended INR and platelet thresholds | INR ≤ 2.0 Platelets ≥ 50 × 103 per μL (50 × 109 per L) | INR ≤ 1.5 Platelets ≥ 50 × 103 per μL | INR ≤ 1.5 Platelets ≥ 50 × 103 per μL |
| How long before procedure a medication should be stopped (based on bleeding risk) | |||
| Apixaban (Eliquis) | One day | Two days | Three days |
| Aspirin | — | — | Five days |
| Clopidogrel (Plavix) | Zero to five days | Five days | Five days |
| Dabigatran (Pradaxa) | One day | Two days | Three days |
| Fondaparinux (Arixtra) | One day | 36 hours | Two days |
| Low-molecular-weight heparin (enoxaparin [Lovenox]) | 12 hours (one dose) | 12 hours (one dose) | 12 to 24 hours (one or two doses) |
| NSAIDs | — | — | One to 10 days* |
| Rivaroxaban (Xarelto) | One day | Two days | Two days |
| Unfractionated heparin (intravenous) | One hour | Four hours | Four hours |
| Unfractionated heparin (subcutaneous) | Four hours | Four hours | Six hours |
| Warfarin (Coumadin) | Three to five days | Five days | Five days |
Placement of Indwelling Catheters and Ports
Central venous catheters and subcutaneous ports offer short- and long-term solutions for the administration of intravenous therapies (Figure 1). Interventional radiology uses ultrasound and fluoroscopic guidance to perform central venous cannulation. Nontunneled catheters may be converted to tunneled catheters later for long-term access.
Venous access methods have variable infection risks (Table 2).4 Nontunneled catheters have the highest infection rate over time (2.7 infections per 1,000 days vs. 1.6 infections for tunneled catheters).4 Infections occur in one-fifth of patients with tunneled catheters because of prolonged placement.4 Subcutaneous ports improve infection rates and are commonly used for recurrent chemotherapy infusions.4 Overall complications and costs are reduced when ports are placed with interventional radiography rather than surgically, with reported savings of more than $1,500 because of more rapid procedure and turnover times.5–7
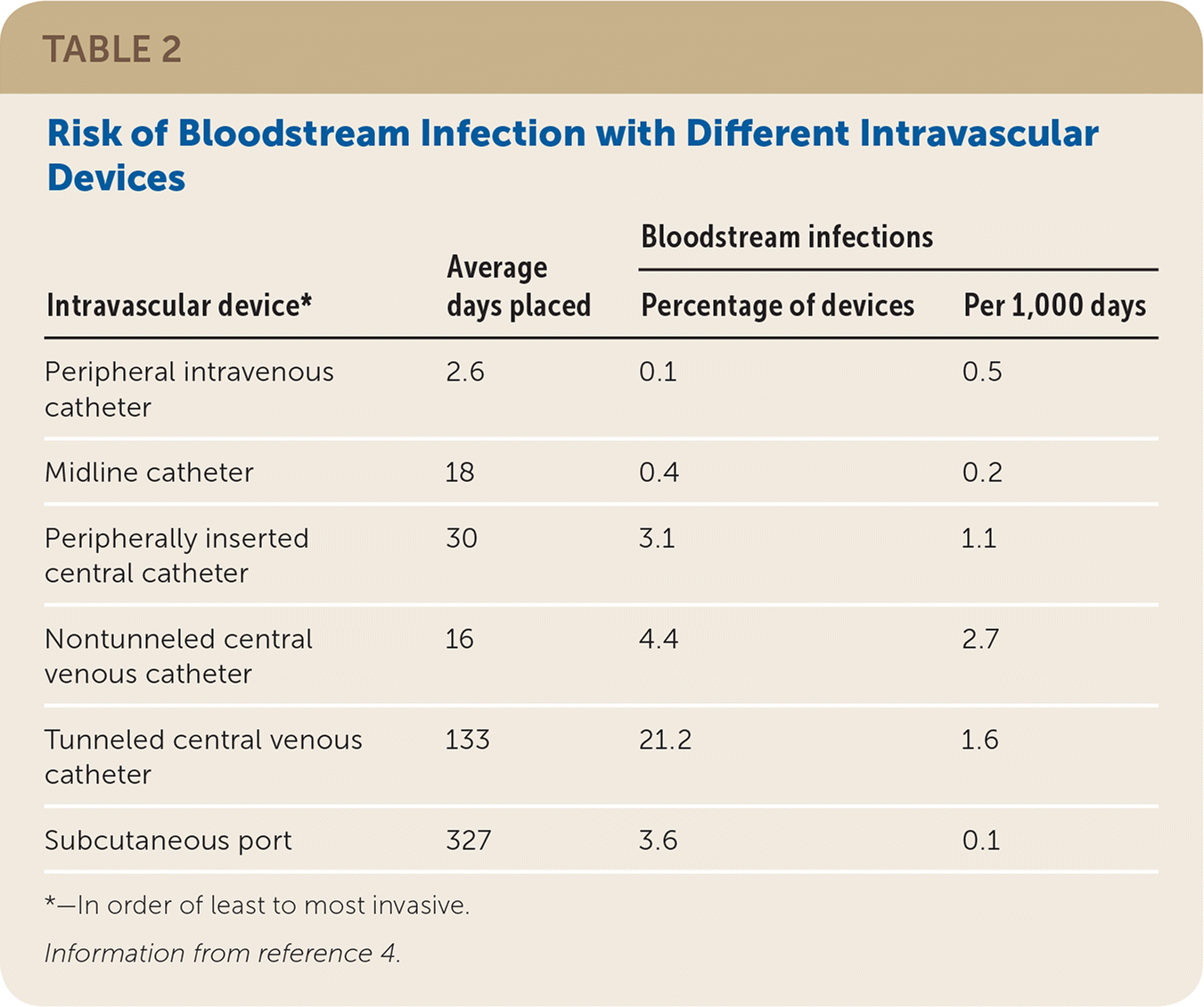
| Intravascular device* | Bloodstream infections | |||
|---|---|---|---|---|
| Average days placed | Percentage of devices | Per 1,000 days | ||
| Peripheral intravenous catheter | 2.6 | 0.1 | 0.5 | |
| Midline catheter | 18 | 0.4 | 0.2 | |
| Peripherally inserted central catheter | 30 | 3.1 | 1.1 | |
| Nontunneled central venous catheter | 16 | 4.4 | 2.7 | |
| Tunneled central venous catheter | 133 | 21.2 | 1.6 | |
| Subcutaneous port | 327 | 3.6 | 0.1 | |
Arterial Interventions
PERIPHERAL ARTERY DISEASE INTERVENTIONS
Peripheral artery disease affects 8.5 million Americans and up to 20% of patients older than 60 years.8 Patients who develop limb ischemia or lifestyle-limiting claudication despite medical therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise antithrombotic medication delivery.
For chronic limb ischemia, a large trial found that angioplasty has lower morbidity, length of hospitalization, and cost but higher mortality than surgical revascularization after two years.9 Therefore, angioplasty is limited to patients with shorter life expectancy.9 Two trials of patients with acute limb ischemia demonstrated identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.10,11 Improvements in mechanical thrombectomy since these trials were conducted may have improved outcomes but have not been compared with surgery.12
AORTIC ANEURYSM REPAIR
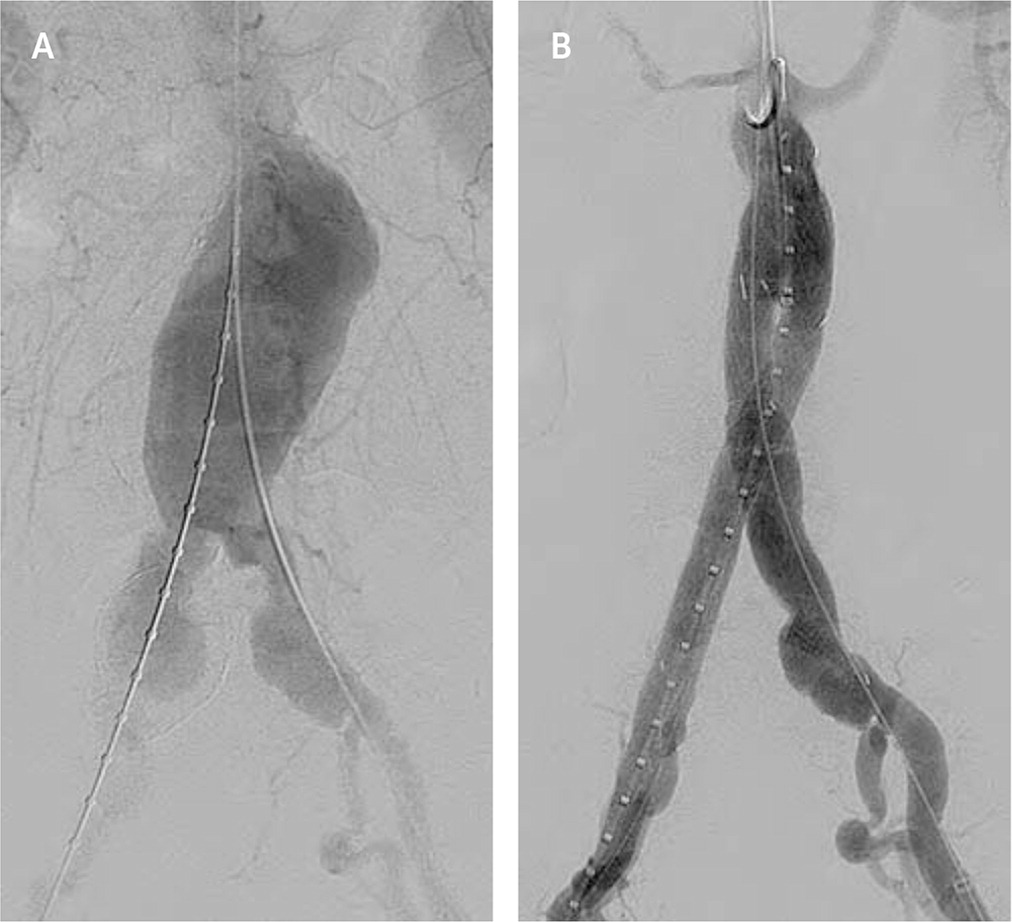
Screening programs have led to increased detection of abdominal aortic aneurysms. Endovascular aortic repair uses fluoroscopic guidance to deploy a metallic stent graft (an impermeable fabric tube supported by a wire stent) to span an aneurysmal segment13 (Figure 2). A systematic review showed that endovascular treatments have improved 30-day mortality and equivalent total mortality compared with surgery.14
Venous Interventions
Chronic venous disease encompasses a wide disease spectrum, with an estimated 22 million women and 11 million men in the United States affected by varicose veins.15 Symptomatic patients with varicose veins that do not respond to conservative management may benefit from endovenous laser therapy, radiofrequency ablation, and sclerotherapy.16 These treatments damage the endothelium, ultimately ablating varicosities. A recent systematic review found comparable long-term outcomes, including varicosity recurrence, in patients who received endovenous laser therapy or radiofrequency ablation vs. surgical intervention for saphenous insufficiency.17 Cosmetic outcomes do not differ between interventional and surgical techniques.15 Fewer complications, including bleeding, infection, and paresthesia, have been observed with endovenous laser therapy compared with surgical high ligation.18
Treatments for Hemorrhage
TRANSARTERIAL EMBOLIZATION
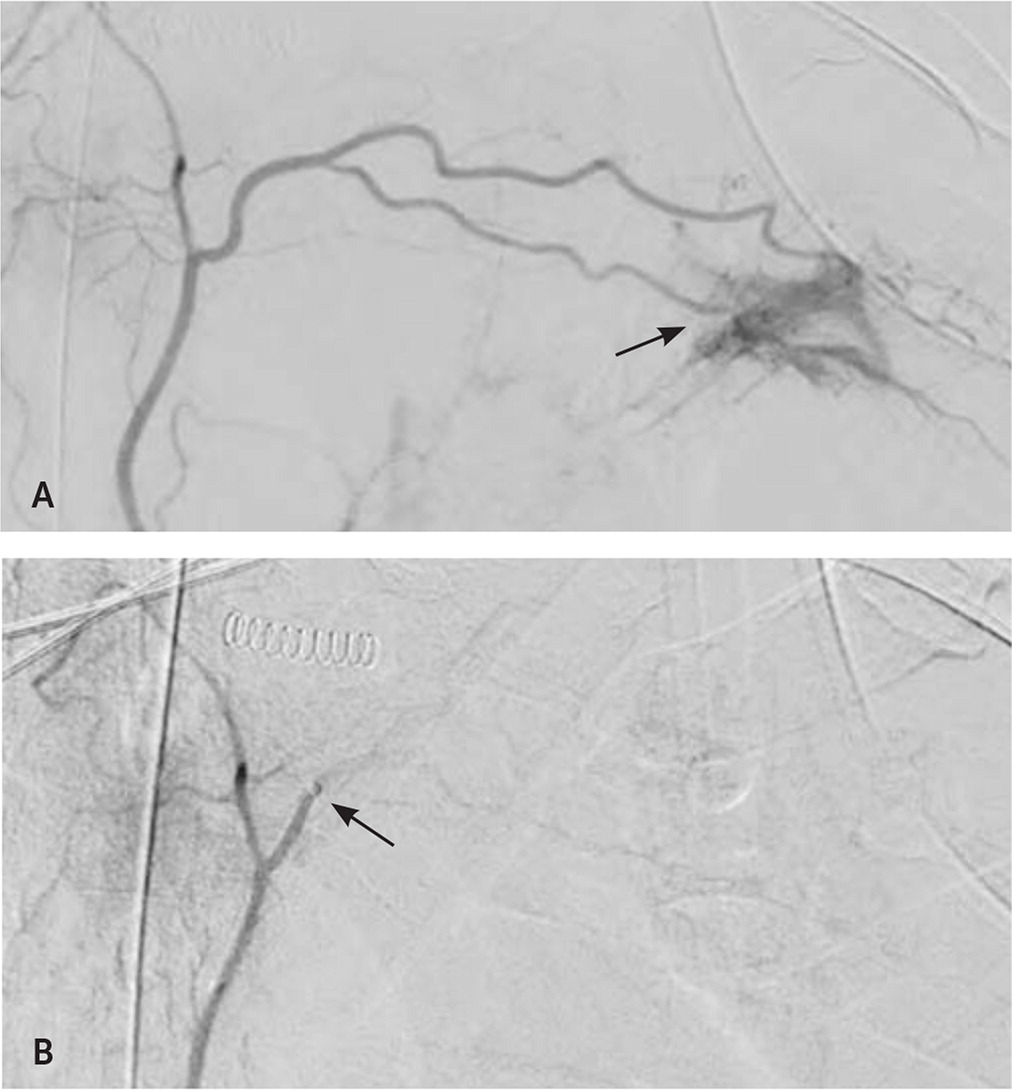
Transarterial embolization involves insertion of hemostatic material through a catheter into a target artery to stop hemorrhage19 (Figure 3). Hemostatic agents include temporary embolic material, such as gelatin sponges, that degrade within days to weeks or more permanent devices, such as platinum coils and polyvinyl alcohol spheres.20 Transarterial embolization is effective for many types of acute hemorrhage (Table 3).21–28 Common complications of the procedure include a postembolization syndrome with transient fever, pain, and nausea. Less common but more severe complications include vessel injury, local necrosis, infection, and venous thromboembolism.29
| Type of hemorrhage | Evidence for transarterial embolization |
|---|---|
| Gastrointestinal tract bleed, lower | 85% to 97% success rate in patients with diverticular bleeds, with 5% to 10% ischemic complications in a meta-analysis of case series21 |
| Gastrointestinal tract bleed, upper | 63% to 97% success rate in small case series22 |
| Hemoptysis | 70% to 99% success rate in a systematic review of observational studies23 |
| Postpartum hemorrhage | 89% success rate in a systematic review24 |
| Postsurgical | Case series show 100% success rate after orthopedic surgery and abdominal anastomosis25,26 |
| Retroperitoneal bleed | 100% success rate in small case series27 |
| Trauma | Trials show greater than 90% success rate in patients with spleen, liver, or kidney injuries and greater than 85% success rate in patients with pelvic fractures in multiple case series28 |
Approximately 1% of pregnancies are complicated by severe postpartum hemorrhage, which causes 14% of pregnancy-related deaths.30,31 Pelvic transarterial embolization can be used to treat severe postpartum hemorrhage. A systematic review of 1,739 cases of severe postpartum hemorrhage showed that transarterial embolization achieved hemostasis in 89% of cases, with 2% of successful procedures occurring after ineffective emergency hysterectomy.24 Up to 12% of patients develop uterine synechiae after transarterial embolization, but the risk is less than with uterine compressive sutures or curettage.32 A systematic review showed a subsequent pregnancy rate of 76% after transarterial embolization, with no increase in miscarriage or intrauterine growth restriction.29 However, these pregnancies have an elevated risk of invasive placental disorders and a nearly 20% risk of postpartum hemorrhage.33
Reproductive Interventions
UTERINE FIBROID EMBOLIZATION
During uterine fibroid embolization, small particles are injected through the uterine arteries after selective catheterization, with the goal of relieving symptoms by shrinking the fibroids.36 A systematic review showed equivalent patient satisfaction and clinical success between uterine fibroid embolization and myomectomy.37 However, these improvements are not always sustained. A Cochrane review showed that up to 32% of patients receiving uterine fibroid embolization require surgical treatment within two years.38
INTERVENTIONS FOR SCROTAL VARICOCELES
Varicoceles affect up to 15% of males and are the most common diagnosis in infertile men.39 Varicoceles are most often treated in cases of orchialgia, infertility, or reduced testicular size in adolescents.39 Endovascular therapy embolizes the affected spermatic vein using coils or sclerosants. Studies have shown that gonadal vein embolization is effective for relieving orchialgia, with 87% of 154 patients having complete pain relief at 39 months in one review.40 A Cochrane review of low-quality studies that did not differentiate between surgery and embolization suggests varicocele treatment improves fertility.41
Treatments for Venous Thromboembolism
CATHETER-DIRECTED THROMBOLYSIS FOR DVT
Postthrombotic syndrome, characterized by limb pain and sensory and skin changes, is an important long-term complication of deep venous thrombosis (DVT). The complication occurs in up to one-half of patients with DVT despite anticoagulation.42 Catheter-directed thrombolysis (Figure 4) delivers thrombolytics to the clot, which can be augmented with endovascular mechanical manipulation and ultrasound-enhanced catheterization.43,44
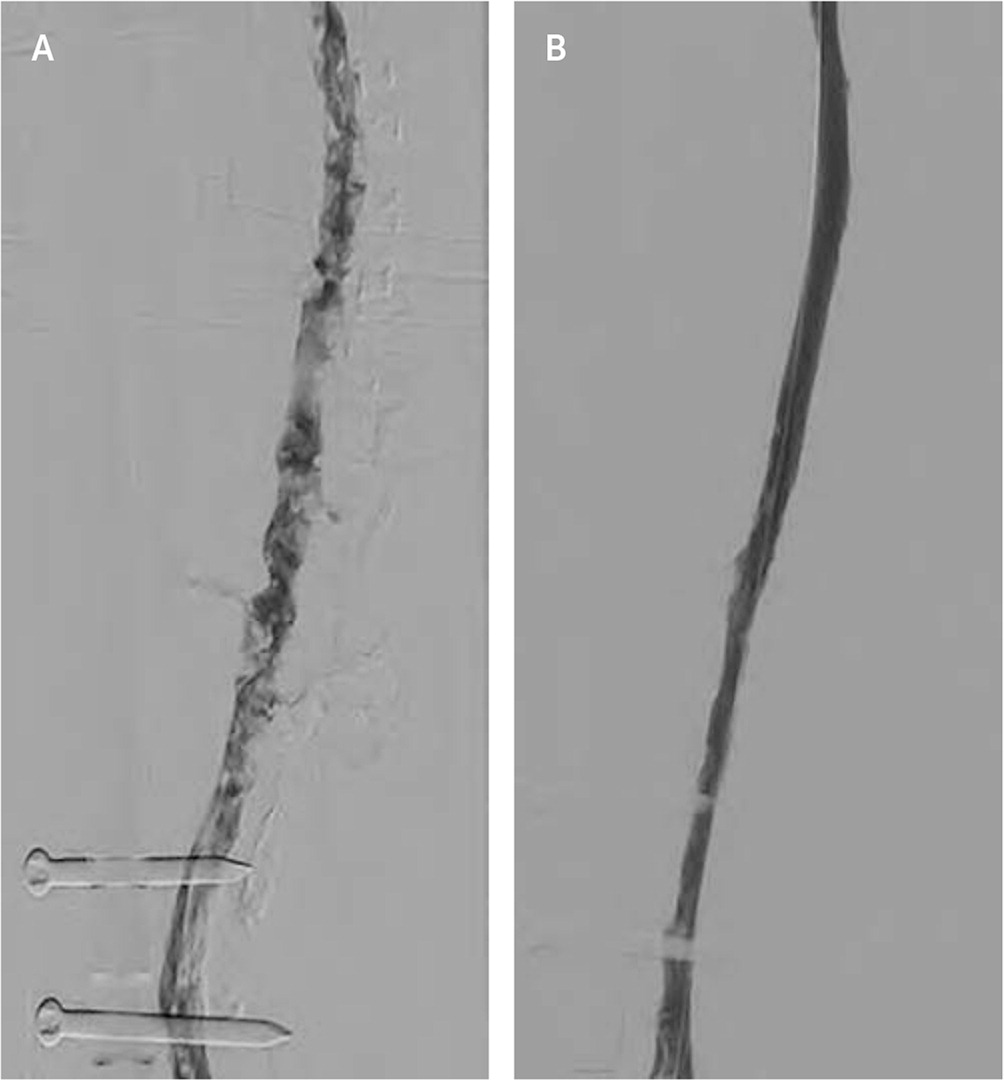
Although the use of thrombolytic therapy has been studied to prevent postthrombotic syndrome in patients with DVT, its effectiveness is uncertain.45 A 2016 Cochrane review showed that catheter-directed thrombolysis has a number needed to treat (NNT) of 7 to prevent one case of postthrombotic syndrome within five years, and a number needed to harm (NNH) of 36 for bleeding.46 However, in a subsequent large, multicenter, randomized trial, catheter-directed thrombolysis did not reduce postthrombotic syndrome compared with anticoagulation after two years but decreased postthrombotic severity.47 Further research is needed to clarify the role of catheter-directed thrombolysis in DVT treatment.
CATHETER-DIRECTED THROMBOLYSIS FOR PE
Catheter-directed thrombolysis has also been studied for the treatment of pulmonary embolism (PE). Current practice guidelines recommend systemic thrombolysis for massive PE.48 However, a meta-analysis of noncontrolled trials favors catheter-directed thrombolysis over systemic thrombolysis, with an NNT of 13 for preventing death and 5 for preventing major complications.49,50 For submassive PE, guidelines primarily recommend anticoagulation, with consideration of catheter-directed thrombolysis.48 Systemic thrombolysis lowers mortality over anticoagulation with an NNT of 65, but it has an NNH of 19 for major bleeding.51 Catheter-directed thrombolysis has the same mortality benefit as systemic thrombolysis with less major bleeding, improving the NNH to 41.51
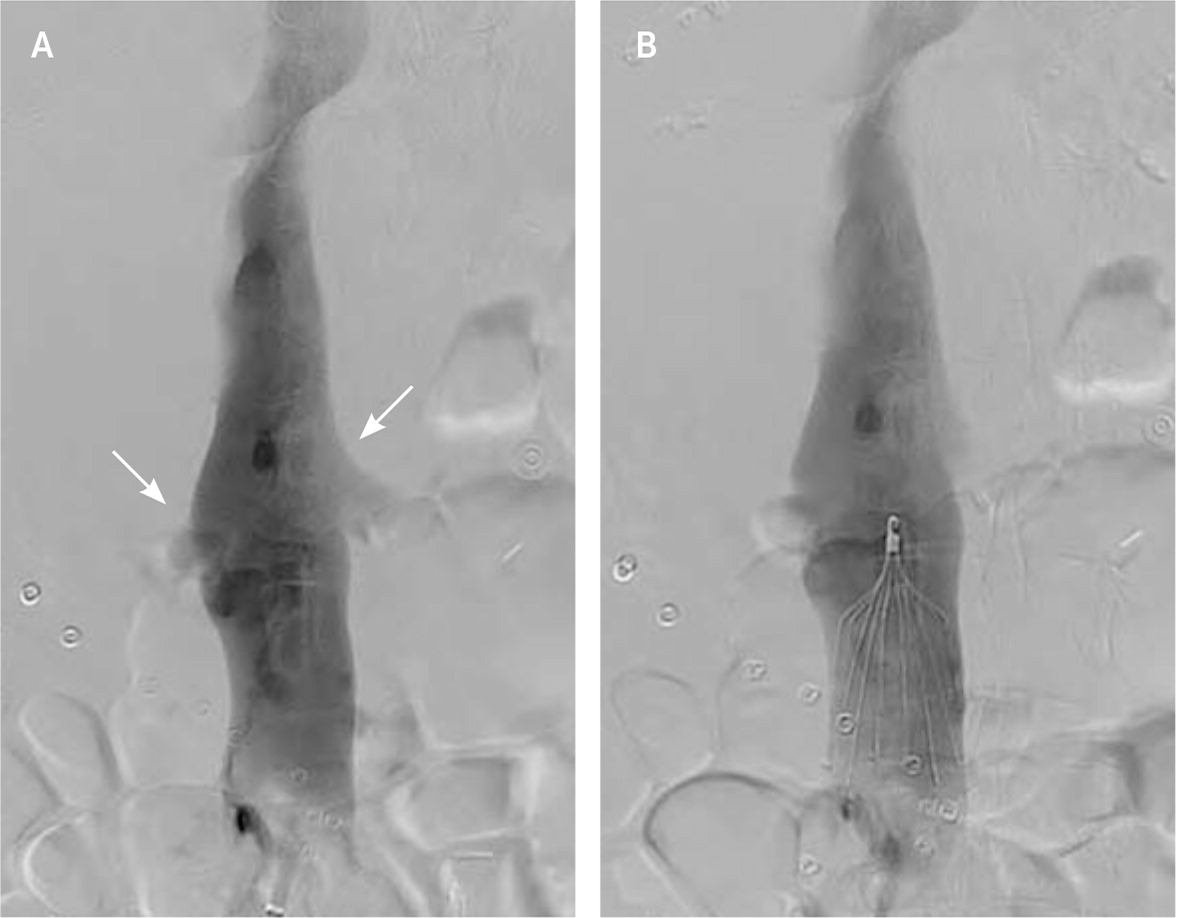
INFERIOR VENA CAVA FILTERS
Inferior vena cava filters are used in up to 13% of patients with venous thromboembolism.52,53 Filters can be placed and retrieved via endovascular approaches (Figure 5). Most guidelines recommend inferior vena cava filters when anticoagulation is contraindicated or PE recurs despite anticoagulation.54 A recent systematic review involving more than 4,000 patients showed that the use of inferior vena cava filters has an NNT of 20 to prevent PE and an NNH of 50 for recurrent DVT.55 There was no difference in absolute or PE-related mortality between patients with and without filters. Because complications from inferior vena cava filters increase over time (Table 4),56 the U.S. Food and Drug Administration issued warnings about increases in adverse effects from inferior vena cava filters, recommending prompt removal when indications allow.53 Inferior vena cava filter placement was reduced after these warnings, yet only 30% of placed filters are removed during the patient’s lifetime.53,54
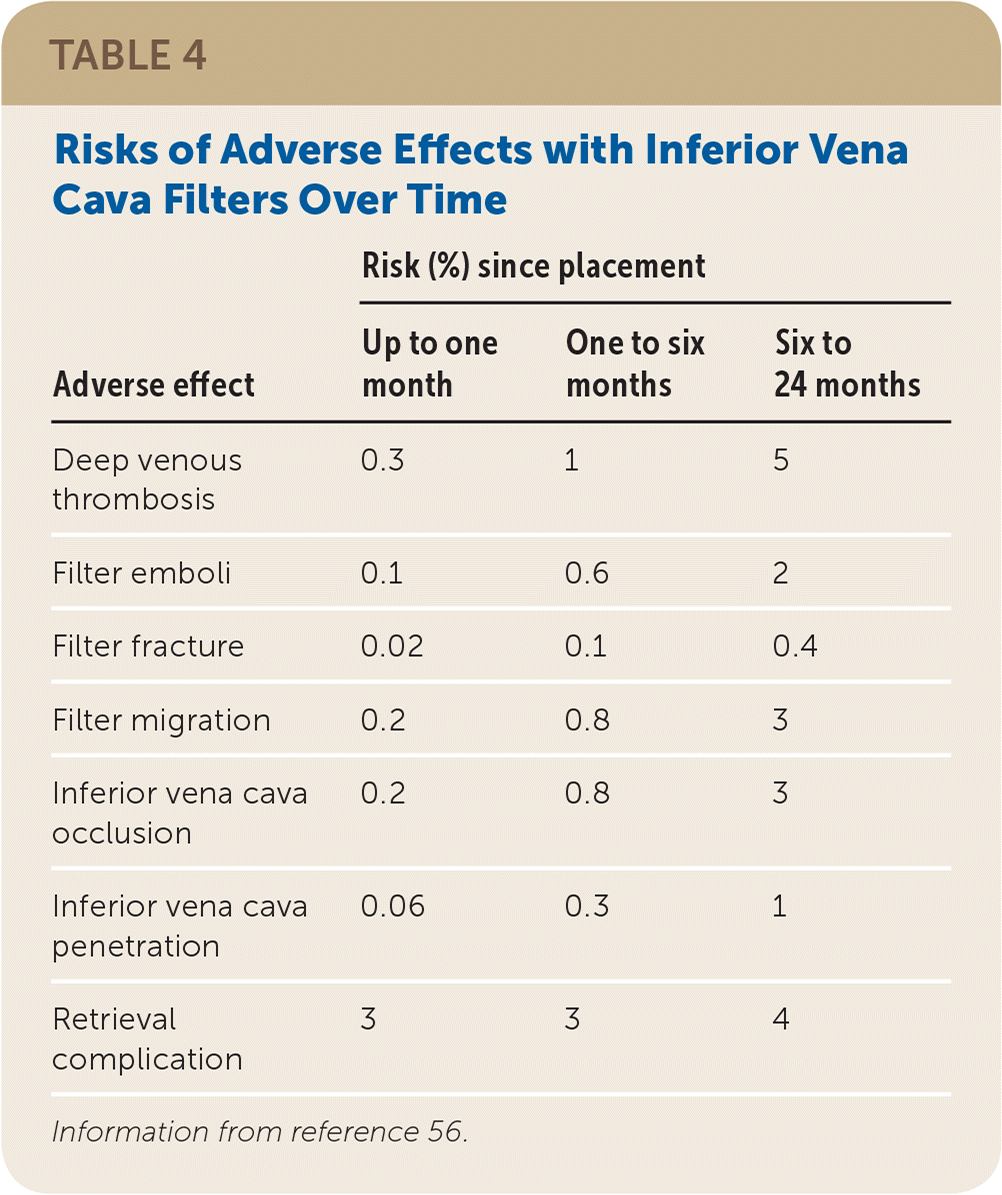
| Adverse effect | Risk (%) since placement | ||
|---|---|---|---|
| Up to one month | One to six months | Six to 24 months | |
| Deep venous thrombosis | 0.3 | 1 | 5 |
| Filter emboli | 0.1 | 0.6 | 2 |
| Filter fracture | 0.02 | 0.1 | 0.4 |
| Filter migration | 0.2 | 0.8 | 3 |
| Inferior vena cava occlusion | 0.2 | 0.8 | 3 |
| Inferior vena cava penetration | 0.06 | 0.3 | 1 |
| Retrieval complication | 3 | 3 | 4 |
Percutaneous Drainage and Biopsy
Image-guided percutaneous drainage and biopsy are safe, well-tolerated procedures and can be performed in nearly any part of the body.57–60 The benefits of percutaneous drainage are well-established and reflected in current practice guidelines.61 Indications include further characterization of abnormal fluid collections, definitive drainage, or partial drainage before definitive surgery.62 Patients with abscesses larger than 3 cm are usually considered candidates for drain placement, whereas patients with smaller abscesses or who need sterile collections can be treated with aspiration or antibiotic therapy alone.63 Overall, the outcomes of percutaneous drainage are at least equivalent to open surgical approaches, with possible reductions in morbidity, length of hospital stay, and cost.64,65
Image guidance can be used for biopsies in a nontargeted fashion or to sample a specific mass. For superficial head and neck masses, such as in the lymph nodes, salivary glands, or thyroid, ultrasonography is commonly used. Guidelines recommend fine-needle aspiration for concerning thyroid lesions, but nearly 30% of samples are nondiagnostic.66,67 Core needle biopsies improve sample adequacy to 95%, although negative predictive values vary from 69% to 93% depending on site.68 Biopsy of deeper head and neck masses often requires computed tomography guidance for optimal visualization to avoid high-risk structures.69 Small studies show that computed tomography–guided biopsies provide adequate samples from 73% to 96% of deep neck lesions.70,71
Liver masses can be biopsied with high accuracy, although rates of needle tract seeding approach 5% in patients with hepatocellular carcinoma.72 Kidney mass sampling is often unnecessary because of high rates of benign and indolent disease but has high accuracy for characterizing indeterminate lesions.73 Computed tomography–guided transthoracic sampling of peripheral lung nodules has a much higher sensitivity than ultrasound-guided transbronchial biopsy, although it has a 1% rate of pneumothorax requiring a chest tube.74 In children, biopsy of soft tissue masses has an accuracy of more than 95%, with most lesions amenable to ultrasound guidance because of proximity to the skin surface.75
Interventional Oncology
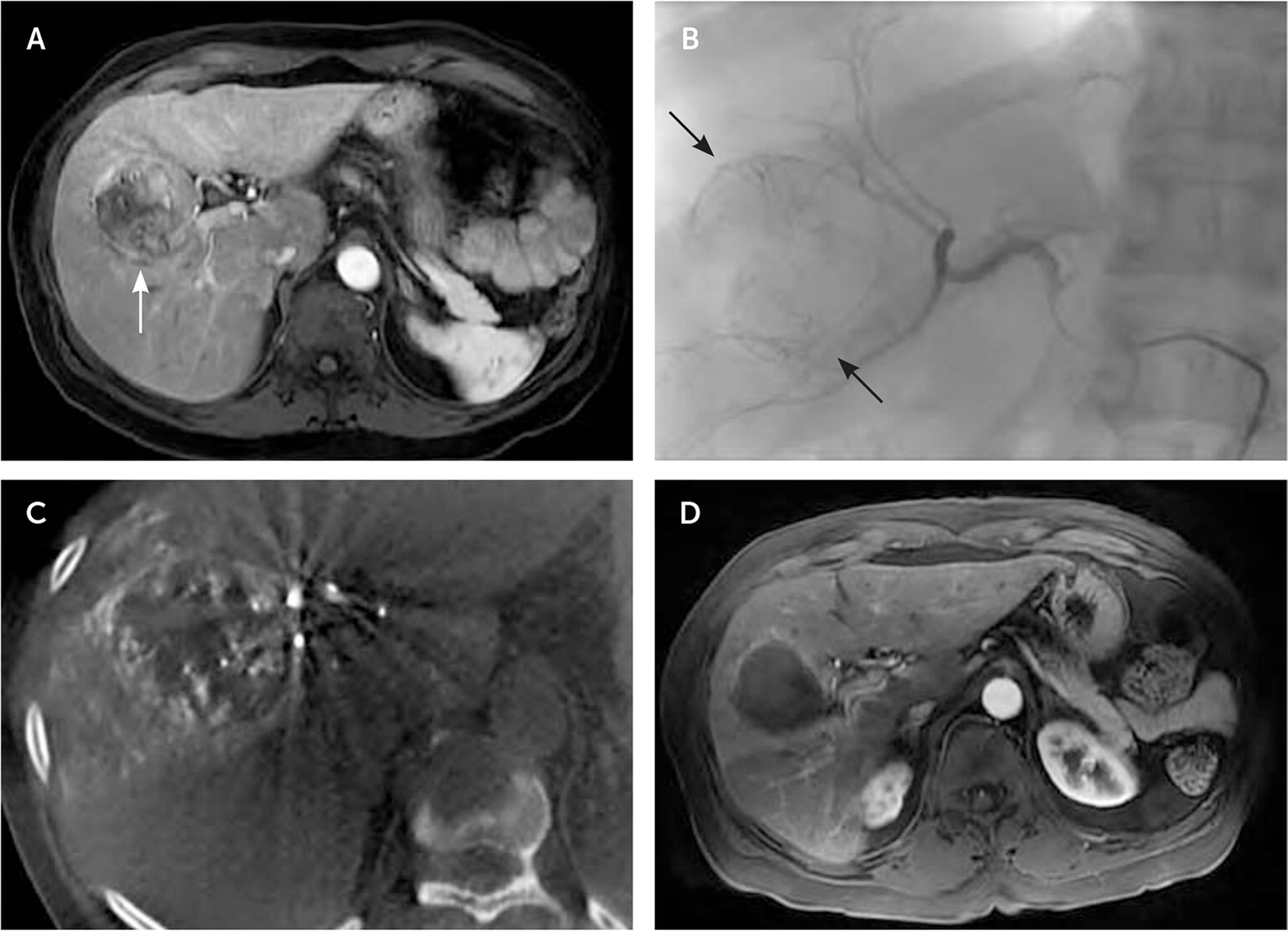
Many interventional radiology techniques are used to treat solid tumors.76 Transarterial therapies include embolization (Figure 6) and targeted delivery of chemotherapy or radiation therapy. Tumors can be ablated by radiofrequency ablation, microwave ablation, cryoablation, or irreversible electroporation. Other interventional treatments can be used to ameliorate mass effects of tumors, including percutaneous drainage or stenting. Table 5 lists common interventional oncology treatments.76
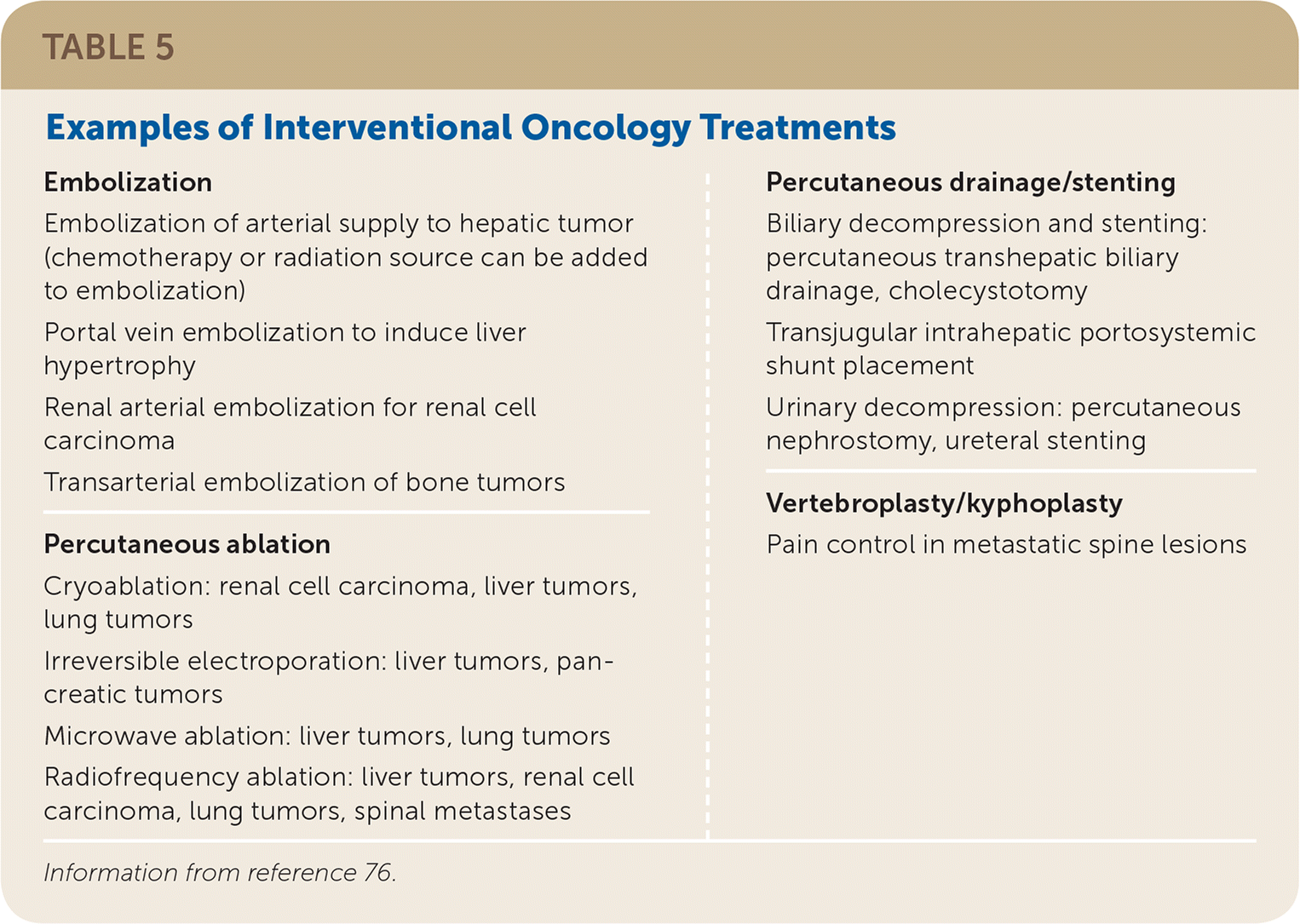
| Embolization | Percutaneous drainage/stenting |
| Embolization of arterial supply to hepatic tumor (chemotherapy or radiation source can be added to embolization) | Biliary decompression and stenting: percutaneous transhepatic biliary drainage, cholecystotomy |
| Portal vein embolization to induce liver hypertrophy | Transjugular intrahepatic portosystemic shunt placement |
| Renal arterial embolization for renal cell carcinoma | Urinary decompression: percutaneous nephrostomy, ureteral stenting |
| Transarterial embolization of bone tumors | |
| Percutaneous ablation | Vertebroplasty/kyphoplasty |
| Cryoablation: renal cell carcinoma, liver tumors, lung tumors | Pain control in metastatic spine lesions |
| Irreversible electroporation: liver tumors, pancreatic tumors | |
| Microwave ablation: liver tumors, lung tumors | |
| Radiofrequency ablation: liver tumors, renal cell carcinoma, lung tumors, spinal metastases |
TREATMENTS FOR BONE METASTASES
Spinal metastases occur in two-thirds of patients with metastatic cancer, and 30% of these patients have significant pain.77 Vertebroplasty involves the percutaneous injection of cement into the affected vertebrae, whereas kyphoplasty adds balloon inflation to restore vertebral height before cement injection. Both techniques are effective, with improvement in short-term pain in more than 90% of patients and functional improvements in more than 60% of patients.78 Reduction in local cancer recurrence has been observed after vertebroplasty, likely secondary to cytotoxic effects and heat from curing cement.79 Asymptomatic extrusion of cement from the vertebral body is common and leads to neurologic or vascular complications in up to 5% of patients.80 These procedures are less likely to benefit patients with osteoporotic vertebral compression fractures.81
LIVER-DIRECTED THERAPIES
The liver is the most common site of cancer metastases, presenting in 48% of metastatic breast cancers and up to 80% of metastatic colon cancers.82 Hepatocellular carcinoma, typically seen with cirrhosis, is less common than metastasis but has high mortality.83 Surgical resection of liver metastases is associated with higher survival than radio-frequency ablation but is often not feasible.84,85 Less than one-half of patients are candidates for resection, even after adjuvant chemotherapy.86 Quality-adjusted survival after radiofrequency ablation appears to be improved over no intervention and over surgery for smaller (less than 3 cm) tumors.86 Other liver-directed therapies can be palliative, such as placement of transjugular intrahepatic portosystemic shunts or biliary drainage procedures.76
Data Sources: PubMed searches were completed using the key terms interventional radiology, catheter-directed thrombolysis, and percutaneous. After the important interventions were identified, searches were performed for each modality. The searches included systematic reviews, meta-analyses, randomized controlled trials, and review articles. We also searched the Cochrane database, Essential Evidence Plus, and Clinical Evidence. In addition, references in these resources were searched. Search dates: January 2018, March 2018, April 2018, and January 2019.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army or Navy, Uniformed Services University of the Health Sciences, Department of Defense, or the U.S. government.