Am Fam Physician. 2019;99(9):558-564
Related letter: Potential Drug Interactions in Patients Taking Oral Contraceptive Pills
Author disclosure: No relevant financial affiliations.
Drug interactions are common in the primary care setting and are usually predictable. Identifying the most important and clinically relevant drug interactions in primary care is essential to patient safety. Strategies for reducing the risk of drug-drug interactions include minimizing the number of drugs prescribed, re-evaluating therapy on a regular basis, considering nonpharmacologic options, monitoring for signs and symptoms of toxicity or effectiveness, adjusting dosages of medications when indicated, and adjusting administration times. Inhibition or induction of cytochrome P450 drug metabolizing isoenzymes is the most common mechanism by which clinically important drug interactions occur. The antimicrobials most likely to affect the international normalized ratio significantly in patients receiving warfarin are trimethoprim/sulfamethoxazole, metronidazole, and fluconazole. An empiric warfarin dosage reduction of 30% to 50% upon initiation of amiodarone therapy is recommended. In patients receiving amiodarone, limit dosages of simvastatin to 20 mg per day and lovastatin to 40 mg per day. Beta blockers should be tapered and discontinued several days before clonidine withdrawal to reduce the risk of rebound hypertension. Spironolactone dosages should be limited to 25 mg daily when coadministered with potassium supplements. Avoid prescribing opioid cough medicines for patients receiving benzodiazepines or other central nervous system depressants, including alcohol. Physicians should consider consultation with a clinical pharmacist when clinical circumstances require the use of drugs with interaction potential.
Drug interactions are estimated to cause approximately 2.8% of all hospitalizations annually in the United States, representing more than 245,000 hospitalizations, costing the health care system $1.3 billion.1 Exact figures are unknown because few studies have highlighted the significance of drug interactions in primary care. Many interactions are theoretical or clinically trivial; however, some may have serious or life-threatening consequences. This review focuses on the most common drug interactions likely to be encountered in the primary care setting.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Software programs that identify potentially serious drug interactions may reduce risk of harm. | C | 2 |
| Pharmacist-directed anticoagulation clinics can identify drug interactions with warfarin (Coumadin) and improve patient outcomes. | B | 3, 5 |
| Medication reconciliation and review by a pharmacist as part of a transition of care process can identify potential drug interactions and may improve patient outcomes. | B | 4 |
| An empiric warfarin dosage reduction of 30% to 50% upon initiation of amiodarone therapy is recommended. | C | 14, 15 |
| Beta blockers should be tapered and discontinued several days before clonidine withdrawal to reduce the risk of rebound hypertension. | C | 39, 40 |
| If the combination is necessary, the spironolactone dosage should be limited to 25 mg per day when coadministered with potassium supplements. | C | 44–46 |
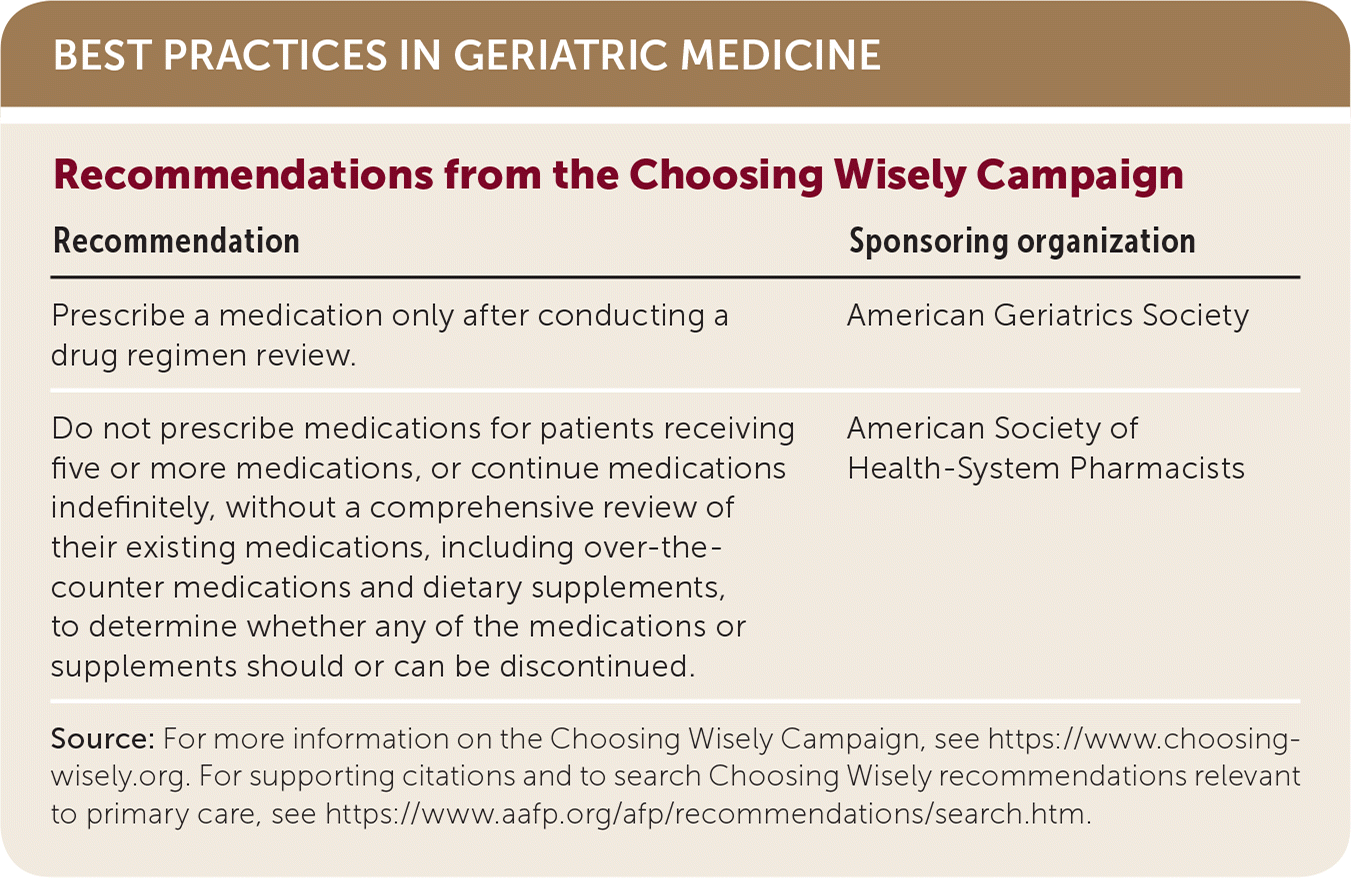
| Recommendation | Sponsoring organization |
|---|---|
| Prescribe a medication only after conducting a drug regimen review. | American Geriatrics Society |
| Do not prescribe medications for patients receiving five or more medications, or continue medications indefinitely, without a comprehensive review of their existing medications, including over-the-counter medications and dietary supplements, to determine whether any of the medications or supplements should or can be discontinued. | American Society of Health-System Pharmacists |
Physicians should be aware of common and serious drug interactions and prescribe to avoid these interactions where possible. Strategies for reducing the risk of drug-drug interactions include minimizing the number of drugs prescribed, re-evaluating therapy on a regular basis, considering nonpharmacologic options, monitoring for signs and symptoms of toxicity and/or effectiveness, adjusting dosages of medications when indicated, and adjusting administration times.1 Physicians should monitor for early detection of adverse reactions and be aware of patient risk factors that increase the chance of an undesirable outcome.
Communication between primary care physicians and subspecialist physicians is critical to mutually understand the goals of drug therapy and to avoid or modify drug combinations that may put patients at risk. Patients should be educated about possible drug interactions when a reaction or potential adverse effect can be anticipated. Electronic drug information resources, as well as software that detects and alerts to a potential drug interaction (often built into electronic prescribing programs), lower the risk of some drug interactions but can cause alert fatigue.2 Medication review and reconciliation by a pharmacist (especially during transitions of care) may reduce the risk of clinically significant drug interactions.3–5 Physicians should consider consultation with a clinical pharmacist when clinical circumstances require the use of drugs with interaction potential.
Mechanisms of Drug-Drug Interactions
Drug-drug interactions can be pharmacodynamic or pharmacokinetic. Pharmacodynamic interactions occur when two drugs that are taken concomitantly have either additive or canceling effects on the body. Pharmacokinetic interactions occur when a drug affects the absorption, distribution, metabolism, or excretion characteristics of another drug.6
Drug interactions based on altered metabolism are mostly attributable to effects on cytochrome P450 (CYP450) isozymes. Inhibition or induction of CYP450 drug metabolizing isozymes is the most common mechanism by which clinically important drug interactions occur. The most common isozyme is CYP3A4, followed by 2C19, 2C9, 1A2, 2E6, and 2D6. Drugs interacting with CYP450 isozymes can be classified as substrates, inducers, or inhibitors. Several drugs are substrates of transport-protein-complexes, such as P-glycoprotein (P-gp), which are expressed in hepatocytes and enterocytes, as well as in the epithelial cells of the renal tubules, the blood brain barrier, and the placenta. These transporters are subject to inhibition or induction that can elicit drug interactions.6
Anticoagulation Interactions
WARFARIN
Warfarin (Coumadin) inhibits vitamin K–dependent clotting factors and is metabolized by CYP450 isozymes. A wide range of drugs, including over-the-counter and herbal products, interact with warfarin.7 Warfarin interactions can increase patients’ risk for major bleeding or thrombotic complications. Vitamin K ingestion modulates warfarin’s effects on coagulation, making a discussion of food-drug interactions with patients essential. Information for patients about warfarin interactions with foods and herbal products can be found at https://www.ihtc.org/warfarin-dietary-tips/.8 Drugs that affect vitamin K (directly or indirectly) or alter warfarin metabolism may have a significant effect on the international normalized ratio (INR). Drugs that affect other parts of the coagulation system (e.g., platelets) when used in conjunction with warfarin may also affect risk of bleeding or thrombosis. Awareness of these interactions is critical to safely managing patients receiving warfarin. Collaborative care with pharmacists in anticoagulation clinics has been demonstrated to improve patient safety and outcomes.3
WARFARIN AND ANTIMICROBIALS
Antimicrobials can inhibit CYP450 isozymes, alter protein binding, and diminish absorption of vitamin K by altering the gut flora.9 Protein-binding and metabolism alterations of warfarin are the mechanisms of interaction that pose clinical significance. The antimicrobials most likely to affect the INR significantly are trimethoprim/sulfamethoxazole, metronidazole (Flagyl), and fluconazole (Diflucan). Other antimicrobials such as ciprofloxacin, levofloxacin (Levaquin), azithromycin (Zithromax), and clarithromycin (Biaxin) may affect the INR; however, the effects are variable and tend to be patient specific.9
Antimicrobial agents with a lower likelihood of affecting the INR include penicillin G benzathine, clindamycin, and first- and fourth-generation cephalosporins.9
Empiric warfarin dosage adjustments should be considered with concomitant use of trimethoprim/sulfamethoxazole, rifampin, fluconazole, and/or metronidazole.10,11 Considerable variability in patient-specific response occurs; knowledge of previous exposure and response should be considered. Regardless of the antimicrobial, the INR should be checked within three to five days of initiation and within three to five days after discontinuation of the antimicrobial.12 Interactions and management strategies for the antimicrobials that consistently interact with warfarin are provided in Table 1.9–12
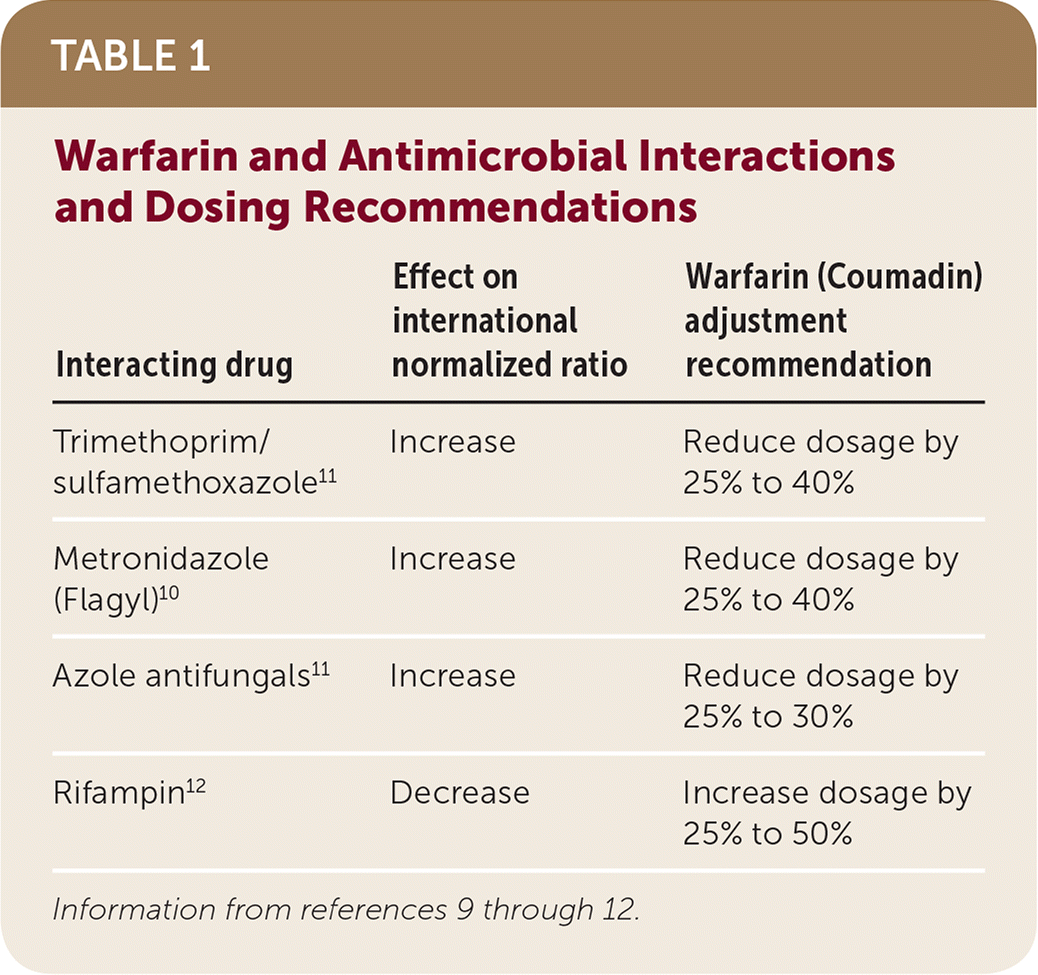
| Interacting drug | Effect on international normalized ratio | Warfarin (Coumadin) adjustment recommendation |
|---|---|---|
| Trimethoprim/sulfamethoxazole11 | Increase | Reduce dosage by 25% to 40% |
| Metronidazole (Flagyl)10 | Increase | Reduce dosage by 25% to 40% |
| Azole antifungals11 | Increase | Reduce dosage by 25% to 30% |
| Rifampin12 | Decrease | Increase dosage by 25% to 50% |
WARFARIN AND AMIODARONE
The interaction between warfarin and amiodarone is mediated by inhibition of CYP2C9, 1A2, and 3A4 enzymes, leading to increased warfarin concentrations and increased bleeding risk.13,14 The effects of this interaction are typically seen within the first few days; however, amiodarone has a long half-life so several warfarin adjustments may be needed before the INR has completely stabilized.15 An empiric warfarin dosage reduction of 30% to 50% upon initiation of amiodarone therapy is recommended, followed by several weeks of weekly monitoring of the INR to ensure warfarin dosing remains optimal.
WARFARIN AND STATINS
Fluvastatin (Lescol), lovastatin (Mevacor), rosuvastatin (Crestor), and simvastatin (Zocor) inhibit warfarin metabolism by the inhibition of CYP2C9, leading to increased warfarin concentrations and increased bleeding risk.16 Atorvastatin (Lipitor) and pravastatin (Pravachol) appear to be safer alternatives. If these statins are not appropriate for treating a patient’s lipids, the patient’s INR should be monitored more frequently, and warfarin dosage adjustments should be performed accordingly.17
WARFARIN AND NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
Nonsteroidal anti-inflammatory drugs (NSAIDs) increase gastric irritation and erosion of the protective lining of the stomach, potentially leading to the formation of gastrointestinal ulcers, which could result in gastrointestinal bleeding. These effects in combination with the anticoagulant effect of warfarin increase the risk of bleeding without affecting the INR.18,19 The cyclooxygenase-2 inhibitor, celecoxib (Celebrex), does not affect platelet function and is associated with less gastrointestinal erosion. Literature correlating gastrointestinal bleeding risk with celecoxib use is limited. Patients should be educated to report abdominal pain or signs and symptoms of gastrointestinal bleeding to their physician when taking warfarin concomitantly with any NSAID.20–22
If concomitant therapy with warfarin and aspirin/dipyridamole (Aggrenox) is necessary, the dosage of aspirin/dipyridamole should be limited to 100 mg per day or less to minimize bleeding risk.18 It is preferable to use alternatives to NSAIDs, such as acetaminophen, topical therapy, or judiciously prescribed opioid analgesics when indicated. Acetaminophen does not alter platelet function, but concomitant use may increase the pharmacodynamic effect of warfarin, increasing the INR. To be cautious, limit the acetaminophen dosage to 2 g per day for no more than seven days and complete more frequent INR testing.18
DIRECT ORAL ANTICOAGULANT INTERACTIONS
Direct oral anticoagulant interactions inhibit factor Xa (rivaroxaban [Xarelto], apixaban [Eliquis], edoxaban [Savaysa]) or directly inhibit thrombin (dabigatran [Pradaxa]).23 Direct oral anticoagulant interactions are attractive alternatives to warfarin for some indications because of their ease of administration, no requirement for INR monitoring, no interaction between vitamin K or food, and comparable effectiveness and safety profiles.24 However, clinically significant interactions occur with drugs that share common metabolic pathways with direct oral anticoagulant interactions.23 Drugs that inhibit both CYP3A4 and P-gp can pose the greatest interaction and increase bleeding risk with direct oral anticoagulant interactions. Drugs that may increase bleeding risk include ketoconazole, ritonavir (Norvir), fluconazole, and amiodarone.23,25 Specific dosing adjustments for the various direct oral anticoagulant interactions are found in Table 2.26–29
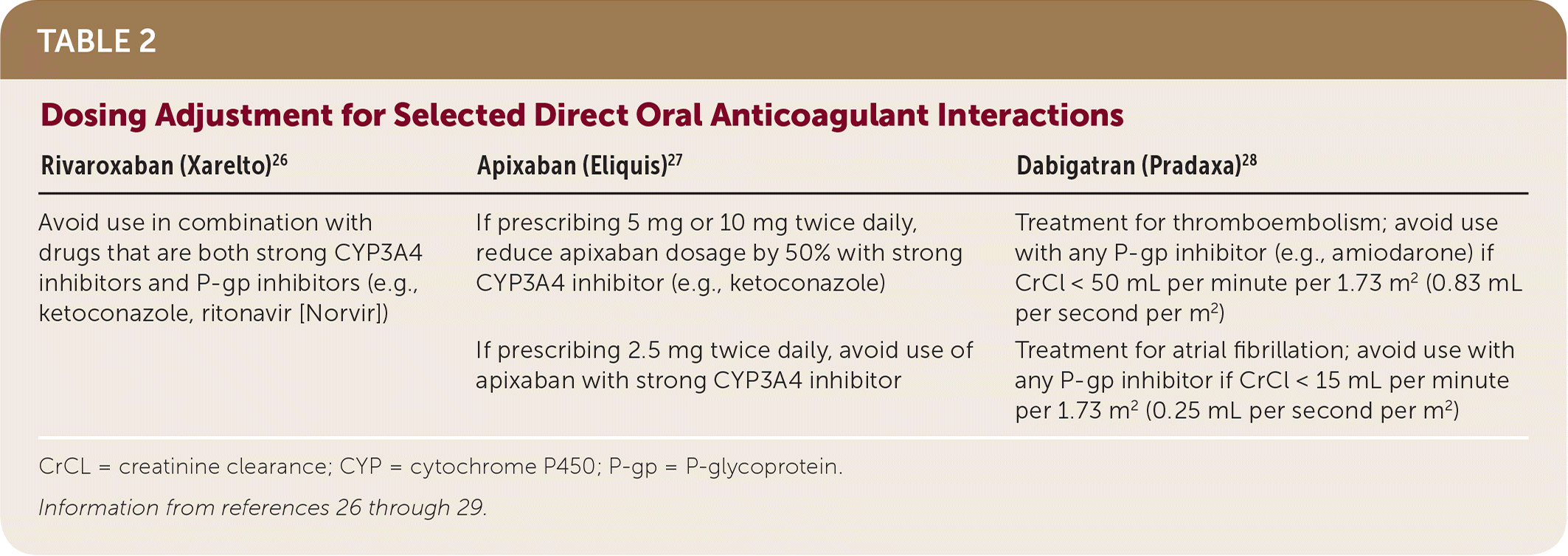
| Rivaroxaban (Xarelto)26 | Apixaban (Eliquis)27 | Dabigatran (Pradaxa)28 |
|---|---|---|
| Avoid use in combination with drugs that are both strong CYP3A4 inhibitors and P-gp inhibitors (e.g., ketoconazole, ritonavir [Norvir]) | If prescribing 5 mg or 10 mg twice daily, reduce apixaban dosage by 50% with strong CYP3A4 inhibitor (e.g., ketoconazole) | Treatment for thromboembolism; avoid use with any P-gp inhibitor (e.g., amiodarone) if CrCl < 50 mL per minute per 1.73 m2 (0.83 mL per second per m2) |
| If prescribing 2.5 mg twice daily, avoid use of apixaban with strong CYP3A4 inhibitor | Treatment for atrial fibrillation; avoid use with any P-gp inhibitor if CrCl < 15 mL per minute per 1.73 m2 (0.25 mL per second per m2) |
Statin Interactions
AMIODARONE
Amiodarone inhibits CYP3A4, which may decrease metabolism of certain statin drugs and increase the risk of statin-related muscle toxicity. Predisposing risk factors for muscle toxicity include age (older than 65 years), obesity, and renal/hepatic impairment.30,31 Fluvastatin and pravastatin are metabolized through alternative pathways that do not affect amiodarone metabolism and are recommended over simvastatin and atorvastatin. When alternative statins are not appropriate, limit dosages of simvastatin to 20 mg per day or lovastatin to 40 mg per day when used concomitantly with amiodarone.31
AZOLE ANTIFUNGALS
Azole antifungals inhibit CYP3A4, which is necessary in the metabolism of simvastatin, lovastatin, and, to a lesser degree, atorvastatin. Azole antifungals also inhibit CYP2C9, which is responsible for the metabolism of fluvastatin. These interactions can lead to an increase in statin concentration, thus increasing the risk of muscle toxicity.32,33 Atorvastatin undergoes less metabolism by CYP3A4, but some cases of myopathy with CYP3A4 inhibitors have been reported.34 Management options include use of rosuvastatin or pravastatin, which are not metabolized by CYP3A4, or use of an alternative antifungal such as terbinafine (Lamisil).35
CALCIUM CHANNEL BLOCKERS
The exact mechanism for the interaction between calcium channel blockers and statin drugs is unknown; however, inhibition of CYP3A4 metabolism may play a role.36 These interactions and management strategies, including specific dosage recommendations with concomitant simvastatin, are found in Table 3.36,37
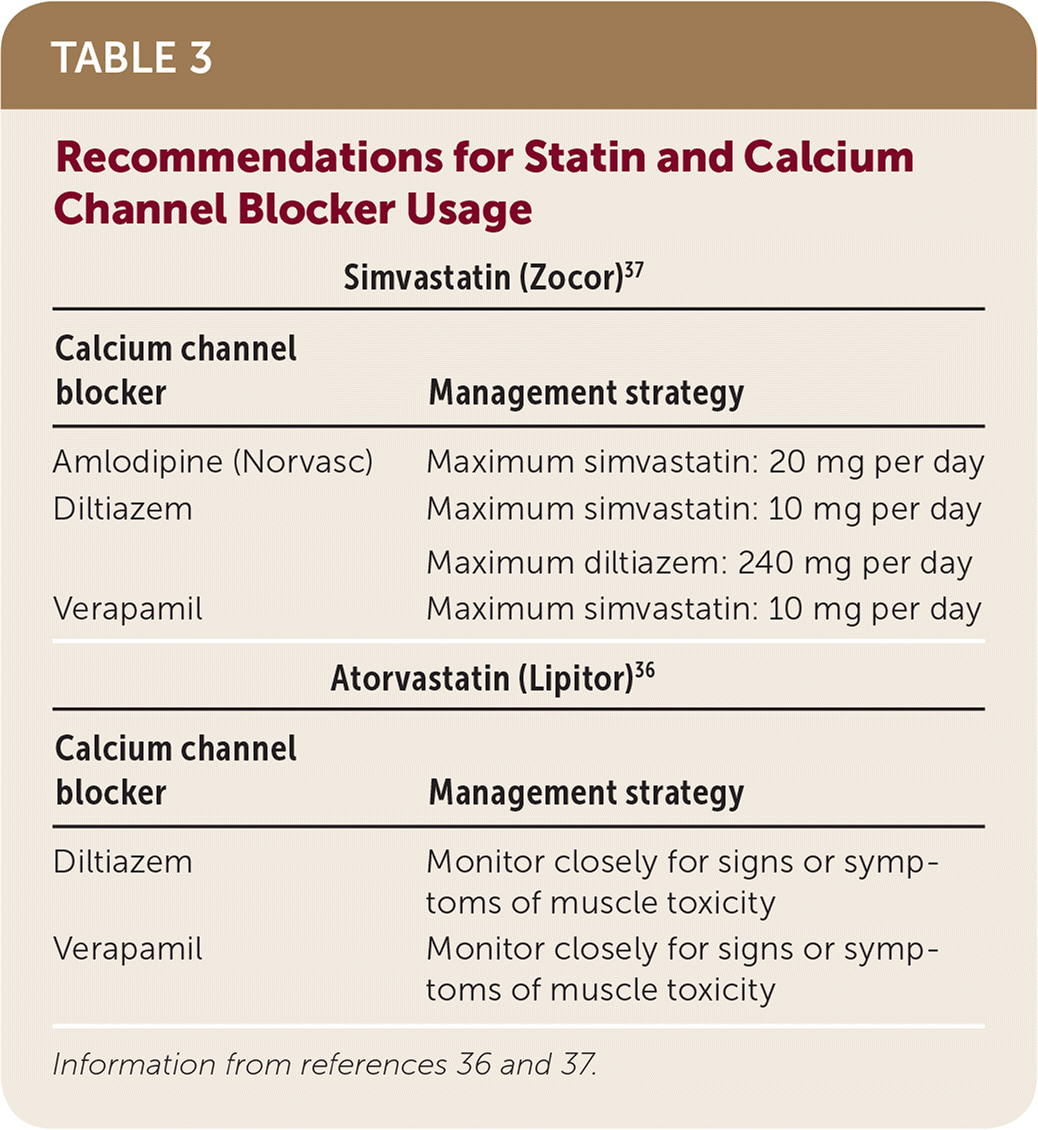
| Simvastatin (Zocor)37 | |
|---|---|
| Calcium channel blocker | Management strategy |
| Amlodipine (Norvasc) | Maximum simvastatin: 20 mg per day |
| Diltiazem | Maximum simvastatin: 10 mg per day |
| Maximum diltiazem: 240 mg per day | |
| Verapamil | Maximum simvastatin: 10 mg per day |
Phosphodiesterase-5 and Nitrates
Phosphodiesterase-5 inhibitors may markedly increase the hypotensive effects of nitrates. Both nitrates and phosphodiesterase-5 inhibitors exert their effects by potentiating the vasodilatory effects of cyclic guanosine monophosphate, which is believed to be responsible for the hypotensive effects when these agents are administered concomitantly.
Phosphodiesterase-5 inhibitors are contraindicated in patients already receiving nitrates. However, 2013 guidelines for management of ST-segment elevation myocardial infarction and unstable angina/non-ST-segment elevation myocardial infarction published by the American College of Cardiology Foundation and American Heart Association support the administration of nitrates when medically necessary at least 24 hours after sildenafil (Viagra) or vardenafil (Levitra) or 48 hours after tadalafil (Cialis).38
Clonidine and Beta Blockers
Several reports describe an increased hypertensive response to the withdrawal of clonidine when a beta blocker is being used.39 In the presence of a beta2-receptor blockade, the vasoconstrictor properties of the catecholamines are unopposed, resulting in the exaggerated hypertensive response within 24 to 72 hours after discontinuation of clonidine.39 Beta blockers should be tapered and discontinued several days before clonidine withdrawal to reduce the risk of rebound hypertension. Recommendations call for decreasing the dosage of clonidine over several days before discontinuation.40
Drugs with Chelation Risk
Chelation occurs when insoluble complexes are formed in the gut as a result of coadministration of multivalent cations and tetracycline drugs, fluoroquinolone antibiotics, or thyroid drugs. When these complexes form, the absorption of these drugs is reduced.18 Managing dosing times for tetracycline antibiotics (administer two hours before or at least four hours after cation41), fluoroquinolone antibiotics (administer two hours before or six hours after cation42), and thyroid drugs (administer two hours before or six hours after cation42) helps to minimize these drug interactions. Many cations are present in over-the-counter products (Table 440). It is important to inquire about the use of over-the-counter medications because patients may not initially report usage.43
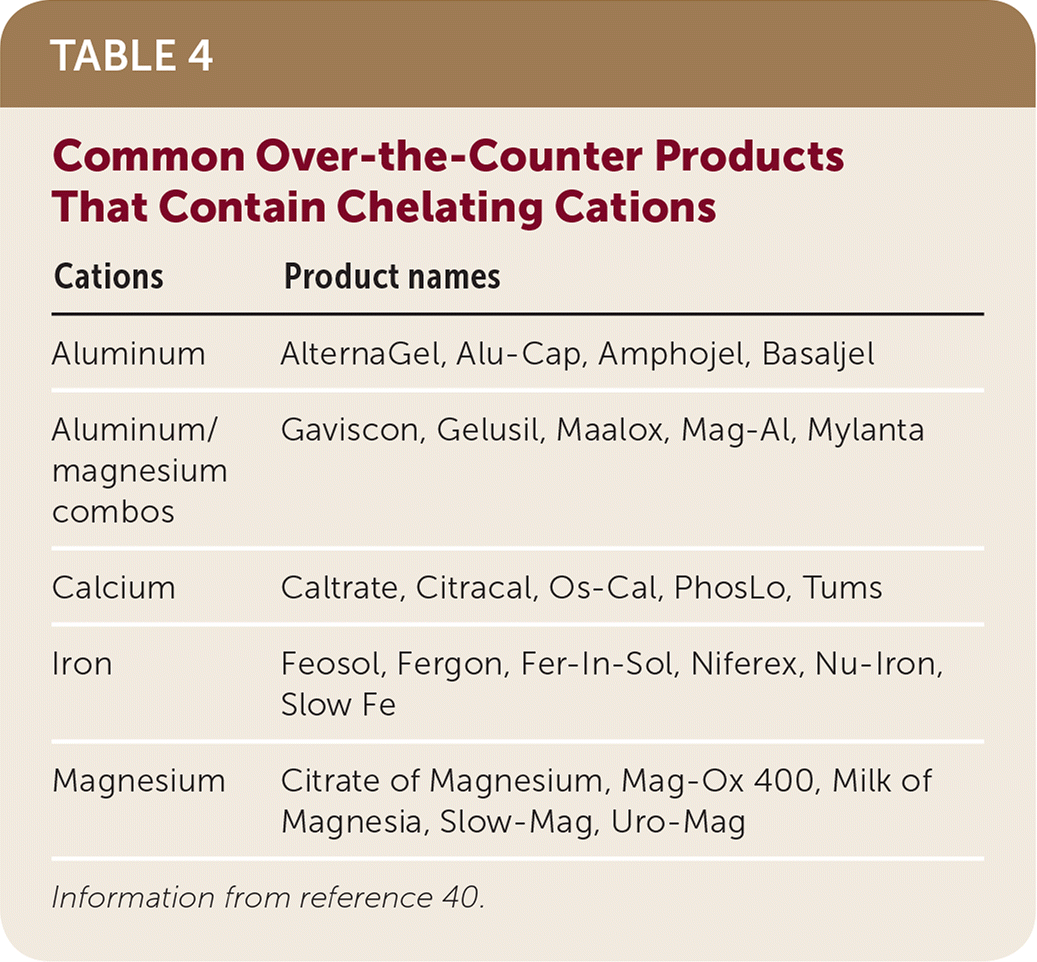
| Cations | Product names |
|---|---|
| Aluminum | AlternaGel, Alu-Cap, Amphojel, Basaljel |
| Aluminum/magnesium combos | Gaviscon, Gelusil, Maalox, Mag-Al, Mylanta |
| Calcium | Caltrate, Citracal, Os-Cal, PhosLo, Tums |
| Iron | Feosol, Fergon, Fer-In-Sol, Niferex, Nu-Iron, Slow Fe |
| Magnesium | Citrate of Magnesium, Mag-Ox 400, Milk of Magnesia, Slow-Mag, Uro-Mag |
Potassium supplements coadministered with angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, direct renin inhibitors, or potassium-sparing diuretics can increase the risk of hyperkalemia. This is considered a pharmacodynamic interaction, and the combination should be used with caution. If the combination is necessary, the spironolactone dosage should be limited to 25 mg daily when coadministered with potassium supplements.44–46 Monitoring should be performed; if serum potassium levels increase to greater than 5.5 mEq per L (5 mmol per L) or renal function worsens, doses of these antihypertensive drugs should be held until the potassium level is less than 5 mEq per L (5 mmol per L) and the renal function normalizes for at least 72 hours.45,46
Combination of Drugs That Prolong the QTc Interval
QT prolongation syndrome increases the risk of torsades de pointes, a life-threatening arrhythmia. Although torsades de pointes is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence (Table 5).47 Torsades de pointes is most likely to occur when two or more QT interval–prolonging drugs are administered to a patient who is already at high risk for arrhythmia.47,48
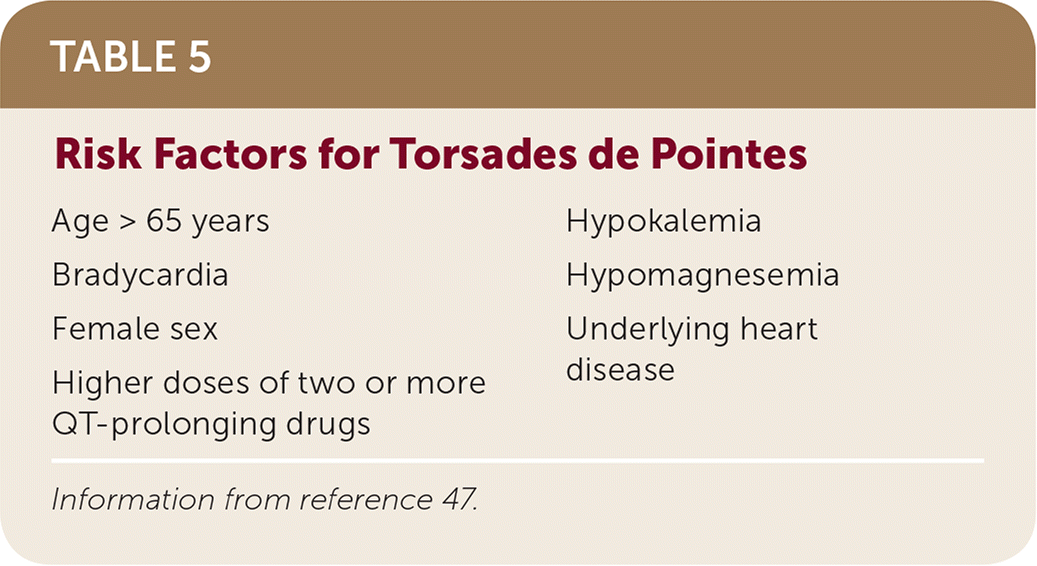
| Age > 65 years | Hypokalemia |
| Bradycardia | Hypomagnesemia |
| Female sex | Underlying heart disease |
| Higher doses of two or more QT-prolonging drugs |
Table 648–51 includes a list of drugs that may cause QT prolongation. Avoiding the combination of drugs from the highest or moderate risk group is advised, especially in patients with a significant history of cardiac events. If unavoidable, monitoring with electrocardiography soon after initiation and yearly thereafter while continuing concomitant therapy is advised.49,51 The U.S. Food and Drug Administration guidance regarding QT prolongation defines increases greater than 20 msec as a substantial risk for developing torsades de pointes.50
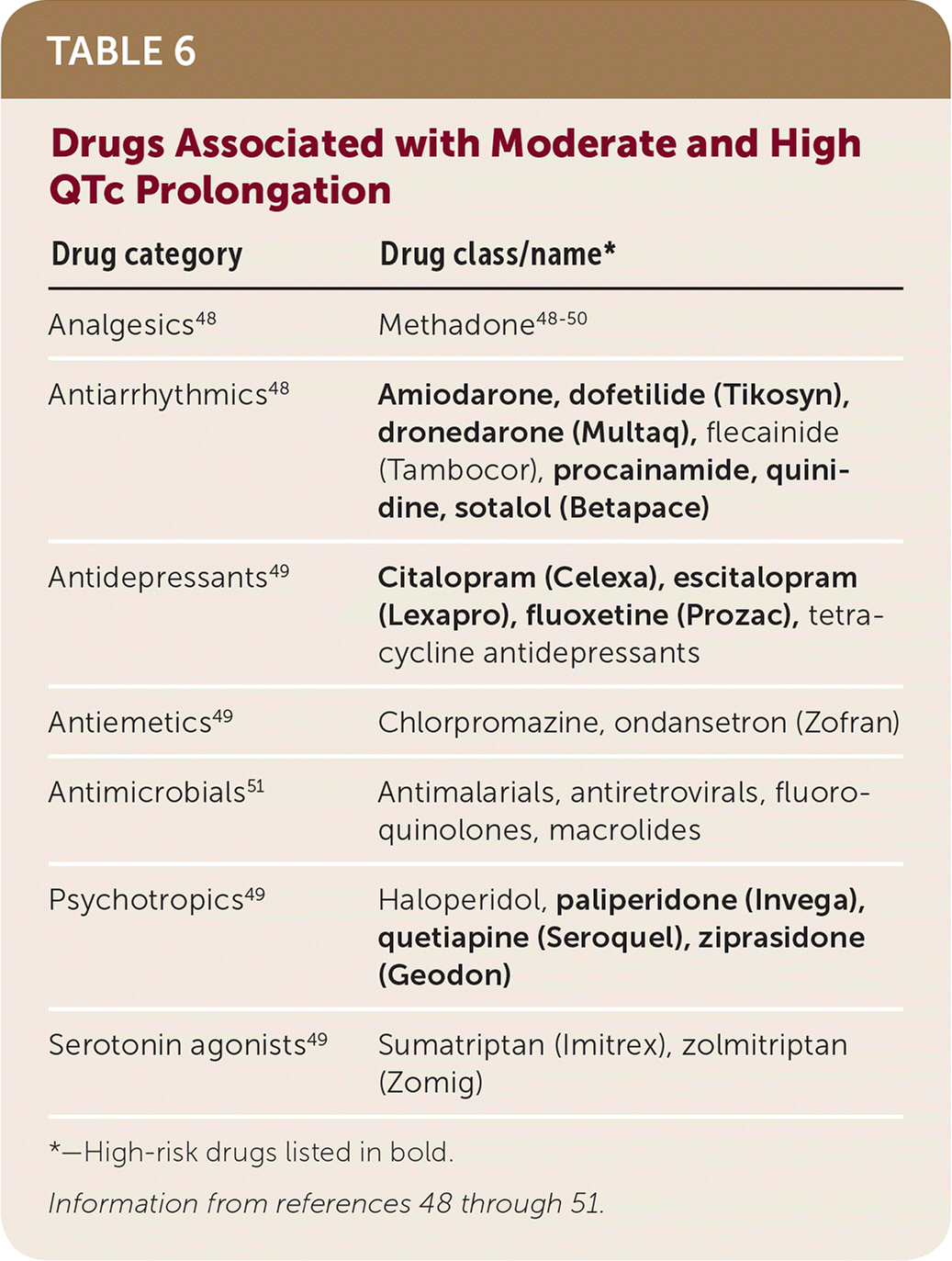
| Drug category | Drug class/name* |
|---|---|
| Analgesics48 | Methadone48–50 |
| Antiarrhythmics48 | Amiodarone, dofetilide (Tikosyn), dronedarone (Multaq), flecainide (Tambocor), procainamide, quinidine, sotalol (Betapace) |
| Antidepressants49 | Citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), tetracycline antidepressants |
| Antiemetics49 | Chlorpromazine, ondansetron (Zofran) |
| Antimicrobials51 | Antimalarials, antiretrovirals, fluoroquinolones, macrolides |
| Psychotropics49 | Haloperidol, paliperidone (Invega), quetiapine (Seroquel), ziprasidone (Geodon) |
| Serotonin agonists49 | Sumatriptan (Imitrex), zolmitriptan (Zomig) |
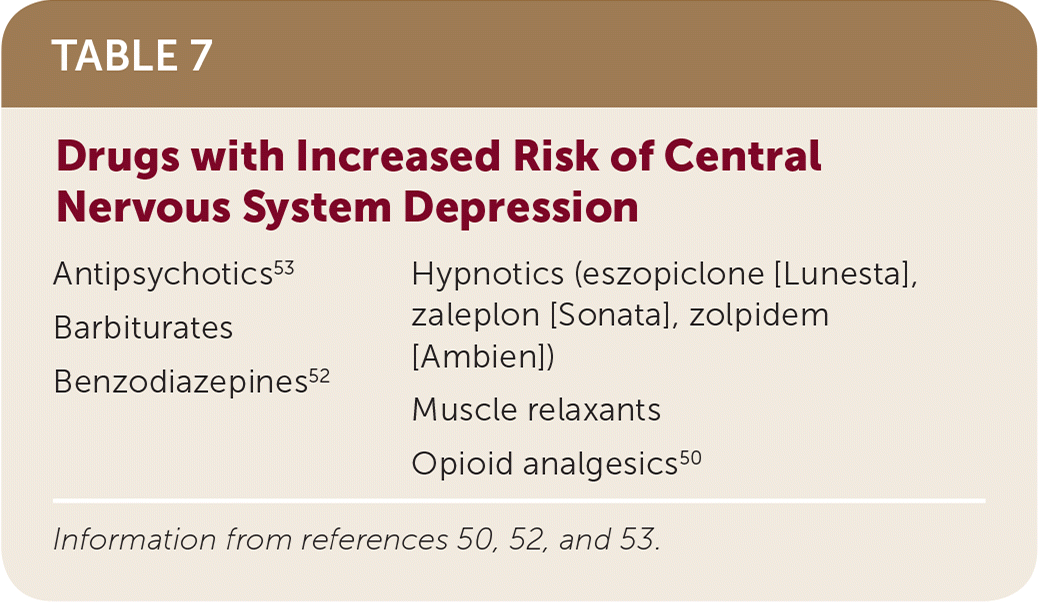
Combination of Central Nervous System Depressing Agents
Health care professionals should limit prescribing medications that can depress the central nervous system. These agents should be combined only if alternative treatment options are inadequate. If these medicines are prescribed together, limiting the dosages and duration of each medication is preferable.52 Prescribing opioid cough medicines for patients receiving benzodiazepines or other central nervous system depressants, including alcohol, should also be avoided.53,54 Patients and caregivers should be educated about signs of central nervous system depression, which include, but are not limited to, slowed or difficulty breathing and/or sedation. A list of drugs predisposing patients to central nervous system depression is provided in Table 7.50,52,53
This article updates a previous article by Ament, Bertolino, and Liszewski.18
Data Sources: We searched PubMed and Web of Science between January 2017 and June 2018, using the search terms drug interaction(s), drug-drug interaction(s), and the MeSH headings drug interactions and food-drug interactions in combination with one or more of the following terms: warfarin, direct oral anticoagulants, novel oral anticoagulants, amiodarone, antimicrobials, antibiotics, statin(s), PDE-5, clonidine, chelator(s), hyperkalemia, QTc prolongation, central nervous system, CNS. The term primary care or the MeSH term primary health care was also employed. Additional resources were identified through Essential Evidence Plus. A literature search using the methods described was performed in June 2018 and January 2019 that limited publication dates between January 2015 through May 2018 and January 2015 through December 2018, respectively, to identify recent publications.