Am Fam Physician. 2019;99(11):676-677
Author disclosure: No relevant financial affiliations.
Clinical Question
What is the preferred anticoagulant for long-term prevention of recurring venous thromboembolism (VTE) in patients with cancer?
Evidence-Based Answer
Low-molecular-weight heparin (LMWH), vitamin K antagonists, and direct oral anticoagulants, when used to prevent recurrent VTE, have a similar impact on all-cause mortality. Compared with vitamin K antagonists, LMWH reduces recurrent VTE in patients with cancer (number needed to treat = 19), with similar adverse event profiles.1 (Strength of Recommendation: A, consistent, good-quality patient-oriented evidence.) Direct oral anticoagulants reduce VTE risk to the same extent as LMWH but at an increased risk of major bleeding (number needed to harm = 34).1 (Strength of Recommendation: B, based on inconsistent or limited-quality patient-oriented evidence.)
Practice Pointers
Patients with cancer have an annual VTE risk of 1.3%, which is six times higher than patients without cancer.2 The risk of VTE recurrence in patients with cancer can reach 29% at one year, leading to recommendations for long-term anticoagulation.3 Cancer also conveys a high risk of major bleeding—up to 20% at one year for patients with both cancer and VTE.4 This Cochrane review evaluated the safety and effectiveness of long-term anticoagulation to prevent VTE recurrence in patients with cancer.1
Sixteen randomized controlled trials involving 5,167 patients with cancer and diagnostically confirmed initial VTE were identified. Patients of all ages with solid or hematologic cancers at any stage were studied. The primary outcome was all-cause mortality; secondary outcomes included recurrent symptomatic VTE and major bleeding. The review evaluated large, multicenter trials and local studies with as few as 35 patients. Most multicenter trials were multinational, whereas the single-center trials were conducted in North America or Europe. Studies varied greatly in the medications used within each class. The larger studies were funded by the sponsoring pharmaceutical company.
Five studies that included 1,781 patients compared LMWH and vitamin K antagonists. There was no difference in mortality between groups. LMWH reduced recurrent symptomatic VTE compared with vitamin K antagonists (number needed to treat = 19; 95% CI, 14 to 34) in patients with cancer, with no difference in major bleeding between the two groups. One study reported no difference in thrombocytopenia between patients receiving LMWH and those receiving vitamin K antagonists.
Four studies with a total of 1,031 patients compared direct oral anticoagulants and vitamin K antagonists and found similar mortality rates, recurrent symptomatic VTE rates, and bleeding events between the groups. Data were low quality because of imprecise reporting and inclusion criteria.
Two studies compared LMWH and direct oral anticoagulants, but only one study, which included 1,016 patients, contained data sufficient for analysis. Mortality risk was similar between the groups, and there was no significant difference in recurrent symptomatic VTE between the direct oral anticoagulant and LMWH treatment arms (relative risk = 0.69; 95% CI, 0.47 to 1.01). Patients using direct oral anticoagulants had an increased risk of major bleeding events compared with patients using LMWH (number needed to harm = 34; 95% CI, 13 to 2,439).
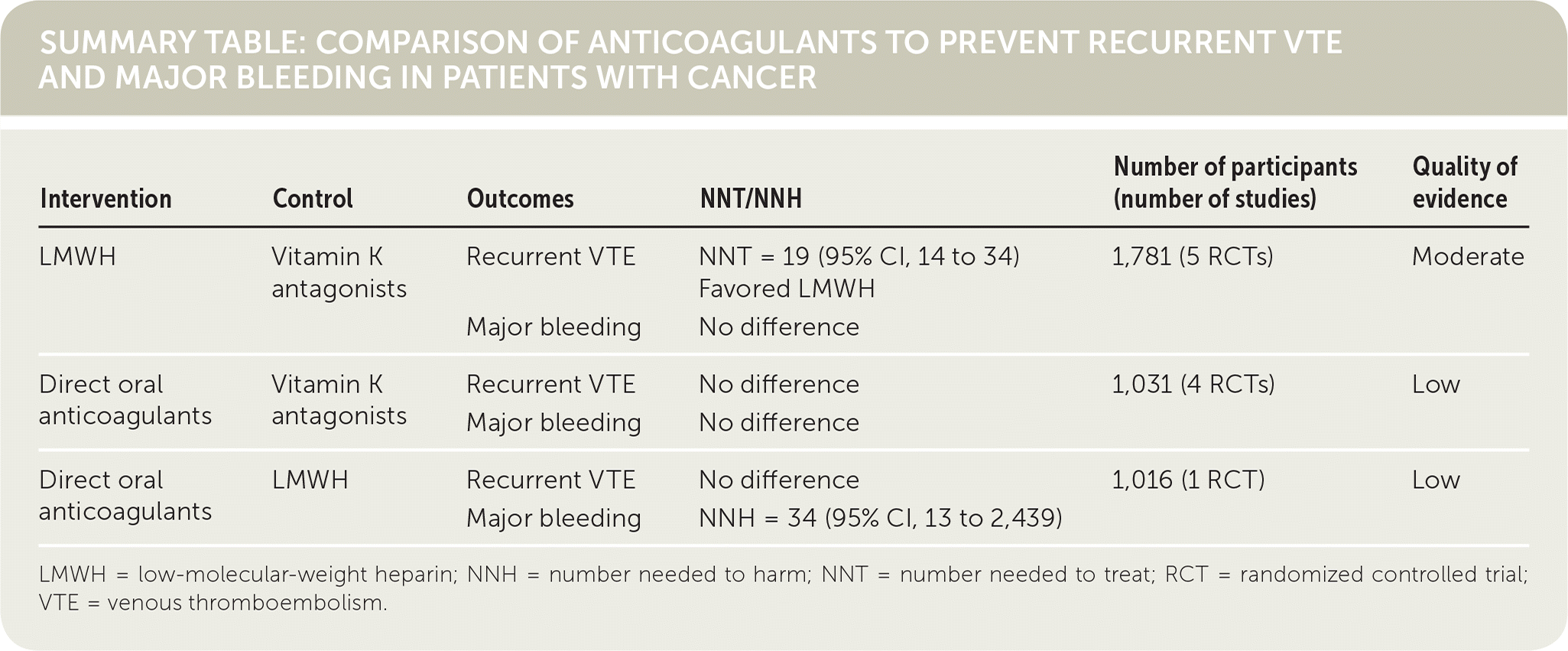
| Intervention | Control | Outcomes | NNT/NNH | Number of participants (number of studies) | Quality of evidence |
|---|---|---|---|---|---|
| LMWH | Vitamin K antagonists | Recurrent VTE | NNT = 19 (95% CI, 14 to 34) Favored LMWH | 1,781 (5 RCTs) | Moderate |
| Major bleeding | No difference | ||||
| Direct oral anticoagulants | Vitamin K antagonists | Recurrent VTE | No difference | 1,031 (4 RCTs) | Low |
| Major bleeding | No difference | ||||
| Direct oral anticoagulants | LMWH | Recurrent VTE | No difference | 1,016 (1 RCT) | Low |
| Major bleeding | NNH = 34 (95% CI, 13 to 2,439) |
A recent systematic review concluded that direct oral anticoagulants do not significantly reduce VTE recurrence compared with LMWH, but they do increase major bleeding.5 Guidelines from the American Society of Clinical Oncology, the American College of Chest Physicians, the European Society for Medical Oncology, and the National Institute for Health and Care Excellence recommend LMWH as first-line treatment for recurrent VTE in patients with cancer, which is consistent with the results of this Cochrane review.1,6–8
The practice recommendations in this activity are available at http://www.cochrane.org/CD006650.
Editor's Note: The numbers needed to treat and to harm, and the corresponding confidence intervals, were calculated by the authors based on raw data provided in the original Cochrane review.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Air Force, Uniformed Services University of the Health Sciences, Department of Defense, or the U.S. government.