Am Fam Physician. 2019;99(12):online
Related Practice Guideline: Depression After ACS Events: AAFP Releases Updated Guidelines.
Author disclosure: No relevant financial affiliations.
Purpose: To review the evidence and provide clinical recommendations for the screening and treatment of depression in patients who have had a recent acute coronary syndrome event.
Methods: This guideline is based on a systematic review of randomized controlled trials and observational studies from January 1, 2003, to August 15, 2017. It is an update to a previous systematic review published in May 2005, which included studies up to March 2004. The target audience for the guideline includes all primary care clinicians, and the target patient population includes adults who are within the three months following an acute coronary syndrome event (unstable angina or myocardial infarction). This guideline was developed using a modified version of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, a transparent approach to evaluating the certainty of the evidence and determining the strength of recommendations.
Recommendation 1: The American Academy of Family Physicians recommends that clinicians screen for depression, using a standardized depression screening tool, in patients who have recently experienced an acute coronary syndrome event (weak recommendation, low-quality evidence). Individuals should undergo further assessment to confirm the diagnosis of depression (good practice point).
Recommendation 2: The American Academy of Family Physicians strongly recommends that clinicians prescribe antidepressant medication, preferably selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, and/or cognitive behavior therapy to improve symptoms of depression in patients who have a history of acute coronary syndrome and have been diagnosed with depression (strong recommendation, moderate-quality evidence).
Guideline Scope and Purpose
The purpose of this guideline is to provide recommendations that are relevant to primary care for the screening and treatment of depression in patients following an acute coronary syndrome (ACS) event. The target audience is family physicians and other primary care clinicians. The target patient population is adults who are within the three months following an ACS event (unstable angina or myocardial infarction).
Differences from Previous Guideline
This guideline updates and replaces the American Academy of Family Physicians (AAFP) guideline on detection and treatment of depression following myocardial infarction, which was published in 20091 and reaffirmed in 2014. The topic was nominated to the Agency for Healthcare Research and Quality (AHRQ) for an updated evidence review in 2015. Changes in methodology and scope from the previous guideline include the following:
Addition of a consumer/patient representative on the guideline panel
Use of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology
Expansion of the target population from patients who have had a myocardial infarction to patients who have had a myocardial infarction or unstable angina
Introduction
Heart disease is the leading cause of mortality in the United States, with more than 600,000 deaths in 2014.2 Coronary heart disease, including ischemic heart disease, is the most common type of heart disease and resulted in approximately 366,000 deaths in the United States in 2015.2 It is estimated that more than 25 million adults in the United States have heart disease and more than 1 million adults are admitted to the hospital with ACS each year.3,4 ACS is defined as the clinical symptoms associated with acute myocardial ischemia and includes unstable angina, non–ST-segment elevation myocardial infarction, and ST-segment elevation myocardial infarction. Patients who have ACS are at increased risk of poor health outcomes, with depression being an important predictor of morbidity and mortality in these patients.5–7
Patients are at an increased risk of mental health disorders following ACS events, including major depressive disorder.8 The prevalence of depression in patients following ACS events is significantly increased compared with the general population. As many as 20% of patients who have ACS were also found to have major depressive disorder, and almost two-thirds of these patients still have symptoms of depression months after the event.9–11 Risk factors associated with the development of depression following ACS events include female sex, low socioeconomic status, type D (distressed) personality, and lack of a support network.9,12,13
Numerous tools have been developed to screen for and diagnose depression. The tools evaluated in patients with a history of ACS include the Beck Depression Inventory-II (BDI-II),14 Patient Health Questionnaire (PHQ),15 Hospital Anxiety and Depression Scale (HADS),16 and Geriatric Depression Scale (GDS).17 These tools vary in number of questions and in scoring systems. All are of easy (third to fifth grade) to average (sixth to ninth grade) literacy level and can be completed by the patient or administered by a health professional. Each of the tools has been validated in the clinical setting. Uncertainty exists about whether clinicians should routinely screen for depression in patients following ACS events. Screening guidelines vary, with some recommending for routine screening, others recommending against routine screening, and some recommending for screening only in patients with additional risk factors.1,18–21
The most common pharmacologic treatments for depression include selective serotonin reuptake inhibitors (SSRIs),22 serotonin-norepinephrine reuptake inhibitors (SNRIs),22 atypical antidepressants,20 and tricyclic antidepressants.20,23 Nonpharmacologic treatments include various forms of psychotherapy, aerobic exercise, education, stress management, transcranial magnetic stimulation, and electroconvulsive therapy. Additionally, enhanced care delivery systems, such as collaborative care, have been evaluated in studies for treatment of depression. Collaborative care is defined as interventions that include systematic, multicomponent, and team-based approaches that ensure patients receive effective medical, preventive, and health maintenance care.24
Methods
SYSTEMATIC REVIEW
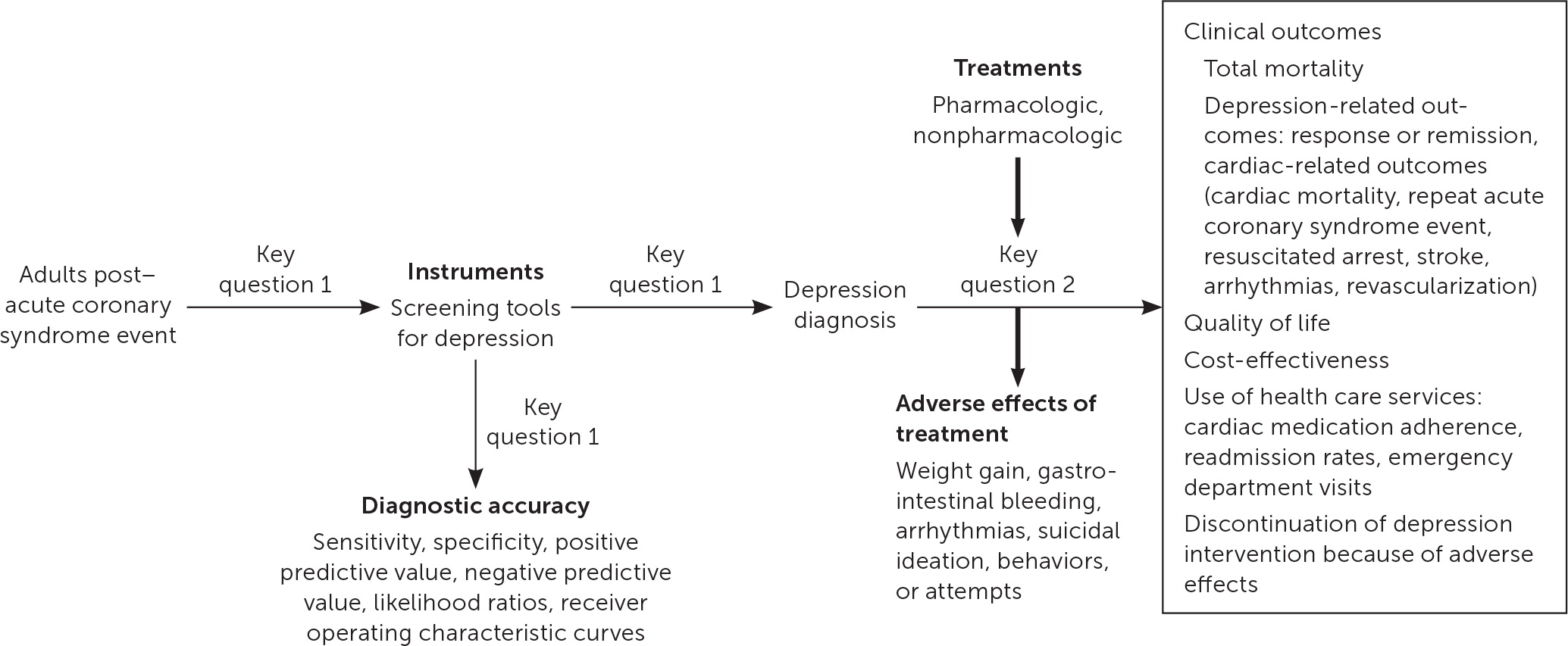
In 2017, AHRQ published comparative effectiveness review no. 200, Diagnostic Accuracy of Screening and Treatment of Post–Acute Coronary Syndrome Depression.20 The report is a systematic review conducted by the Duke University Evidence-Based Practice Center and updates AHRQ report no. 123 from 2005, Post-Myocardial Infarction Depression.25 The report was updated to include recent evidence for the accuracy of depression screening tools and effectiveness of treatments for depression. The target population was expanded to include individuals with unstable angina in addition to individuals with myocardial infarction. The Evidence-Based Practice Center worked with key informants and a technical expert panel to update the previously published report.25 Key informants consisted of identified stakeholders and included physician and patient representatives. The technical expert panel included clinicians with expertise in relevant disciplines such as mental health, cardiology, and family medicine. The updated report included the following key questions and was represented by the analytic framework in Figure 120:
Key Question 1: What is the accuracy of depression screening instruments or screening strategies compared with a validated criterion standard in post-ACS patients?
Key Question 2: What are the comparative safety and effectiveness of pharmacologic and nonpharmacologic depression treatments in post-ACS patients?
Constructing the Guideline
The AAFP's Commission on Health of the Public and Science appointed a guideline development group to update the guideline. Members of this group included physicians with expertise in guideline development, family medicine, and internal medicine, in addition to a patient representative. Specifics on the guideline development process can be found in the AAFP Clinical Practice Guideline Manual.26 The guideline development group reviewed the evidence from the AHRQ evidence report and used a modified version26 of the GRADE27 system to rate the quality of the evidence for each outcome and the overall strength of each recommendation. The “strength of recommendation” reflects the extent to which one can be confident that the desirable effects of an intervention outweigh the undesirable effects and reflect the degree to which there is evidence of improved patient-oriented health outcomes (Table 1).
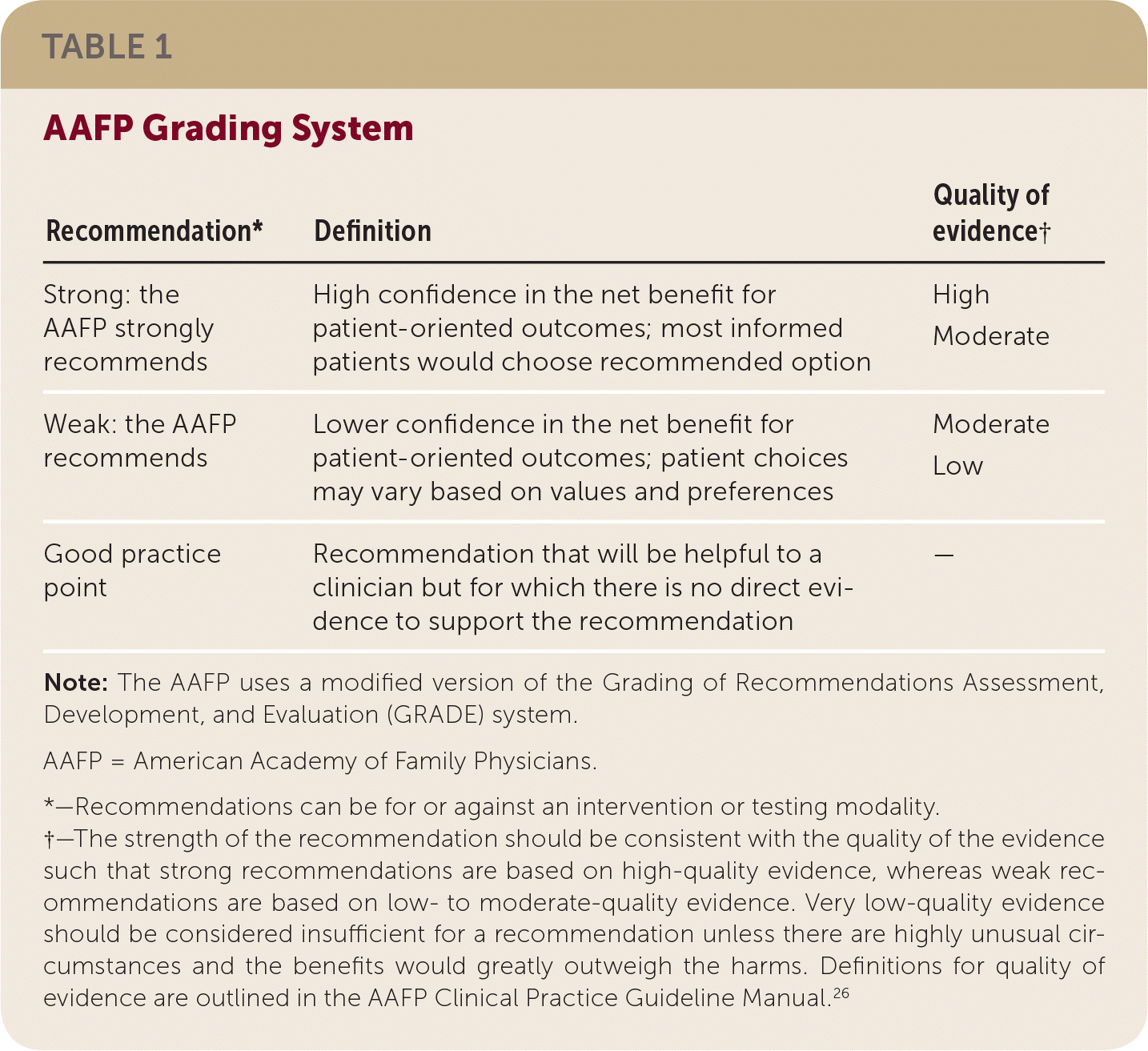
| Recommendation* | Definition | Quality of evidence† |
|---|---|---|
| Strong: the AAFP strongly recommends | High confidence in the net benefit for patient-oriented outcomes; most informed patients would choose recommended option | High Moderate |
| Weak: the AAFP recommends | Lower confidence in the net benefit for patient-oriented outcomes; patient choices may vary based on values and preferences | Moderate Low |
| Good practice point | Recommendation that will be helpful to a clinician but for which there is no direct evidence to support the recommendation | — |
Patient-oriented outcomes were prioritized in the guideline recommendations and included quality of life, adverse events, depressive symptoms, and cardiac outcomes. For Key Question 1, the outcomes assessed included the sensitivity and specificity of depression screening tools. The recommendations were worded to reflect the strength and direction of the recommendation, and the quality of the evidence was listed parenthetically. Guideline recommendations were finalized based on consensus of the guideline development group.
Patient Perspective
Patient preferences and perspective were sought during all stages of guideline development. AHRQ routinely includes patients in the development of evidence reviews to provide input on the scope and key questions. Additionally, all AHRQ evidence reviews are available for public review and comment before being finalized. AAFP includes at least one guideline development group member who is a patient, caregiver, or consumer representative. This member participates throughout the guideline process and is a voting member of the panel. In addition to providing insight into patient preferences during development of the recommendations, the member assists in developing the supporting text for the guideline.
Peer Review
The guideline was peer-reviewed by relevant internal and external stakeholders. All comments and any modifications based on those comments were documented. The AAFP's Commission on Health of the Public and Science and Board of Directors reviewed and approved the final version of the guideline.
Conflict of Interest
The AAFP considers both financial and intellectual conflicts of interest (COI). COIs were solicited in writing at the beginning of the guideline process and updated verbally at each subsequent call. No panel member disclosed any COI. Policies for disclosures and management of COIs are outlined in the AAFP Clinical Practice Guideline Manual.26
Guideline Updating
All AAFP guidelines are scheduled for review five years after completion. Guidelines are reviewed at a shorter interval if new evidence becomes available. This process is managed through the Commission on Health of the Public and Science. Following review, the AAFP determines if the guideline should be reaffirmed, updated, or deleted from the website. All current guidelines developed by the AAFP are available to the public at https://www.aafp.org/patient-care/browse/type.tag-clinical-practice-guidelines.html.
Recommendations
RECOMMENDATION 1
The AAFP recommends that clinicians screen for depression, using a standardized depression screening tool, in patients who have recently experienced an ACS event (weak recommendation, low-quality evidence). Individuals should undergo further assessment to confirm the diagnosis of depression (good practice point).
The AAFP recommends screening for depression in the general adult population.29 Adults who have a negative screening result should be rescreened based on risk factors, comorbid conditions, and life events. ACS is a comorbid condition that is associated with an increased prevalence of depression8–11 and can also be a significant life event. There are tools available to accurately screen for depression in patients following ACS events; for those who are ultimately diagnosed with depression, treatment improves symptoms of depression. Thus, the AAFP recommends that clinicians screen for depression in patients who experience ACS events, even if they previously screened negative.
In its previous guideline, the AAFP recommended that “patients having a myocardial infarction should be screened for depression using a standardized depression checklist…,” despite insufficient evidence to adequately assess the performance characteristics of the screening tools.1 The updated systematic review found adequate evidence that the performance characteristics of depression screening tools in individuals following ACS events are comparable to those in the general population.
The review included six observational studies that evaluated four screening instruments: BDI-II, GDS, HADS, and PHQ. The BDI-II was evaluated in four of the six studies and, therefore, has the most data supporting its use in patients following ACS events. The six studies included a total of 1,755 patients but had a relatively low prevalence of major depressive disorder. In five studies, the screening was done in the inpatient setting, and one study was conducted in a cardiac rehabilitation facility. However, each of the tools can easily be implemented in the outpatient setting. All instruments produced generally acceptable sensitivity, specificity, and negative predictive values but had low positive predictive values. Because of the low positive predictive value, it is recommended that diagnosis follow a two-step process, with further assessment following a positive screening result to confirm the presence of depression.
The BDI-II studies indicated a sensitivity of 90% and specificity of 80%. Although the BDI-II has the most data, it takes the longest amount of time to complete (five to 10 minutes). The HADS was evaluated in three of the six studies, and its performance was similar to the BDI-II, with a slightly lower sensitivity. There was one study each of the PHQ and GDS. The PHQ study included the PHQ-2 and PHQ-9, both of which performed similarly in the post–ACS event population and in the general population. The GDS, which has been validated for individuals 65 years and older, was also similar to the BDI-II, with slightly higher specificity and positive predictive value. The evidence supports a preferential recommendation for use of the BDI-II because of the limited data for the other tools. However, it is reasonable to base the choice of screening tool on availability, clinician comfort, and ease of use. Table 2 provides a comparison of the different screening tools.15–17,20,30
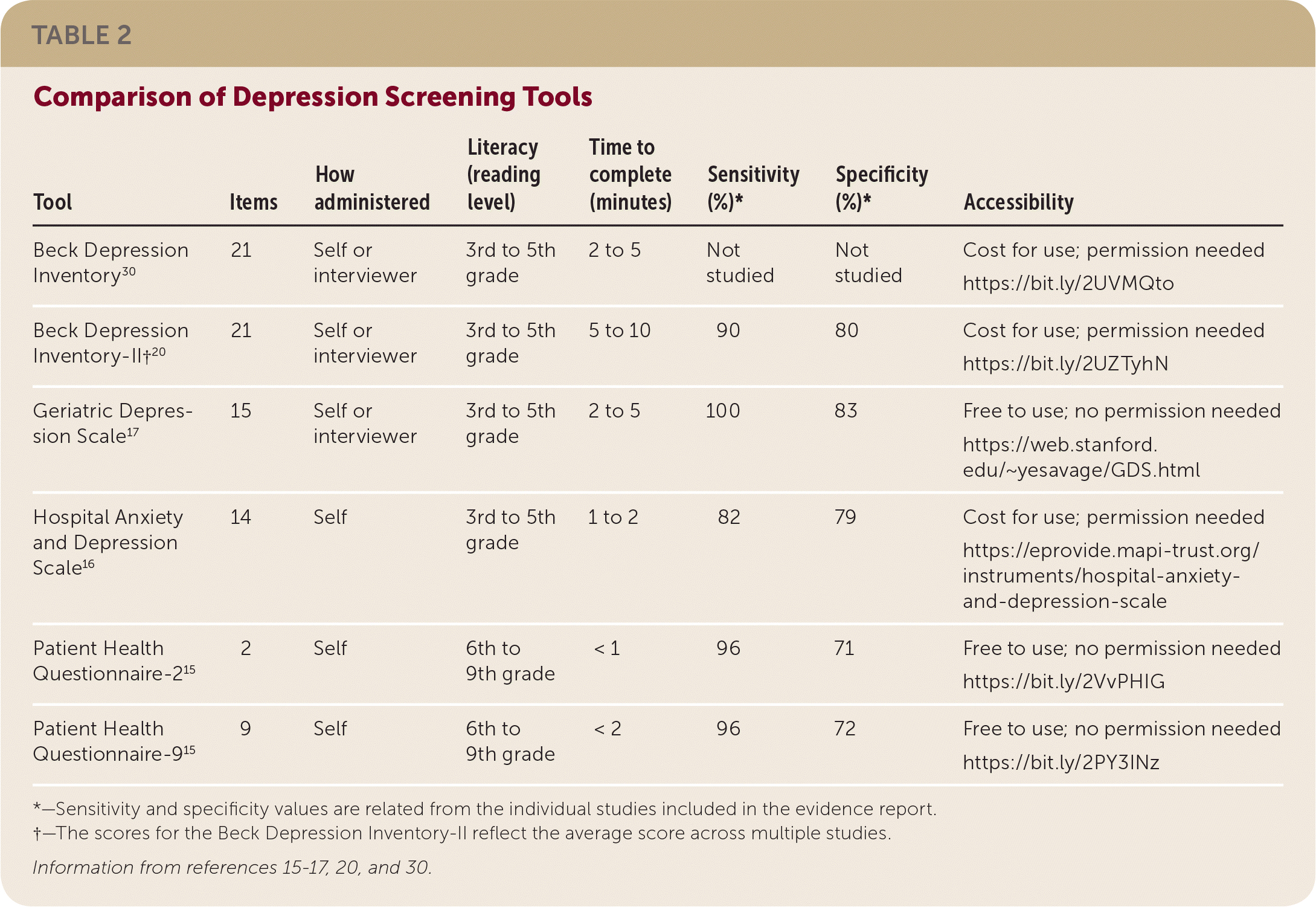
| Tool | Items | How administered | Literacy (reading level) | Time to complete (minutes) | Sensitivity (%)* | Specificity (%)* | Accessibility |
|---|---|---|---|---|---|---|---|
| Beck Depression Inventory30 | 21 | Self or interviewer | 3rd to 5th grade | 2 to 5 | Not studied | Not studied | Cost for use; permission needed https://bit.ly/2UVMQto |
| Beck Depression Inventory-II†20 | 21 | Self or interviewer | 3rd to 5th grade | 5 to 10 | 90 | 80 | Cost for use; permission needed https://bit.ly/2UZTyhN |
| Geriatric Depression Scale17 | 15 | Self or interviewer | 3rd to 5th grade | 2 to 5 | 100 | 83 | Free to use; no permission needed https://web.stanford.edu/~yesavage/GDS.html |
| Hospital Anxiety and Depression Scale16 | 14 | Self | 3rd to 5th grade | 1 to 2 | 82 | 79 | Cost for use; permission needed https://eprovide.mapi-trust.org/instruments/hospital-anxiety-and-depression-scale |
| Patient Health Questionnaire-215 | 2 | Self | 6th to 9th grade | < 1 | 96 | 71 | Free to use; no permission needed https://bit.ly/2VvPHIG |
| Patient Health Questionnaire-915 | 9 | Self | 6th to 9th grade | < 2 | 96 | 72 | Free to use; no permission needed https://bit.ly/2PY3INz |
RECOMMENDATION 2
The AAFP strongly recommends that clinicians prescribe antidepressant medication, preferably SSRIs or SNRIs, and/or cognitive behavior therapy (CBT) to improve symptoms of depression in patients who have a history of ACS and have been diagnosed with depression (strong recommendation, moderate-quality evidence).
Moderate-quality evidence shows that treatment of depression in patients with a history of ACS can improve symptoms of depression, reaffirming the AAFP's 2009 recommendation to treat patients who have depression following a myocardial infarction.1 The evidence of benefit is strongest for a combination of CBT and medication, but the choice of treatment should be based on patient preference, access to services, and clinical judgment. All trials included in the AHRQ report screened patients for depression, and those with a confirmed diagnosis of depression were randomized to the intervention or usual care. Usual care meant that the patient's primary care clinician was informed of the diagnosis of depression, so a significant number of these patients received some form of treatment for their depression, potentially blunting the intervention effect sizes.
There are limited comparative effectiveness data for antidepressant medications to treat depression following ACS events.20 The studies in this evidence review used a variety of medications, including bupropion (Wellbutrin), citalopram (Celexa), escitalopram (Lexapro), mirtazapine (Remeron), sertraline (Zoloft), and venlafaxine (Effexor). The previous evidence review for the AAFP's 2009 guideline on post–myocardial infarction depression1 found high-quality evidence to recommend the use of SSRIs over tricyclic antidepressants. Tricyclic antidepressants have multiple adverse effects, including potential cardiotoxicity, and therefore should not be used in patients with heart disease. Medications used to treat depression in the included studies, with information on approximate cost and adverse effects, are listed in Table 3. The adverse effects listed suggest the complicated trade-offs patients and physicians must evaluate when using pharmacologic solutions to address depression. Insomnia, fatigue, and drowsiness can exacerbate depression. Dizziness, tachycardia, blurred vision, and headache may replicate cardiovascular symptoms and create anxiety in addition to depression for patients who have had an ACS event.
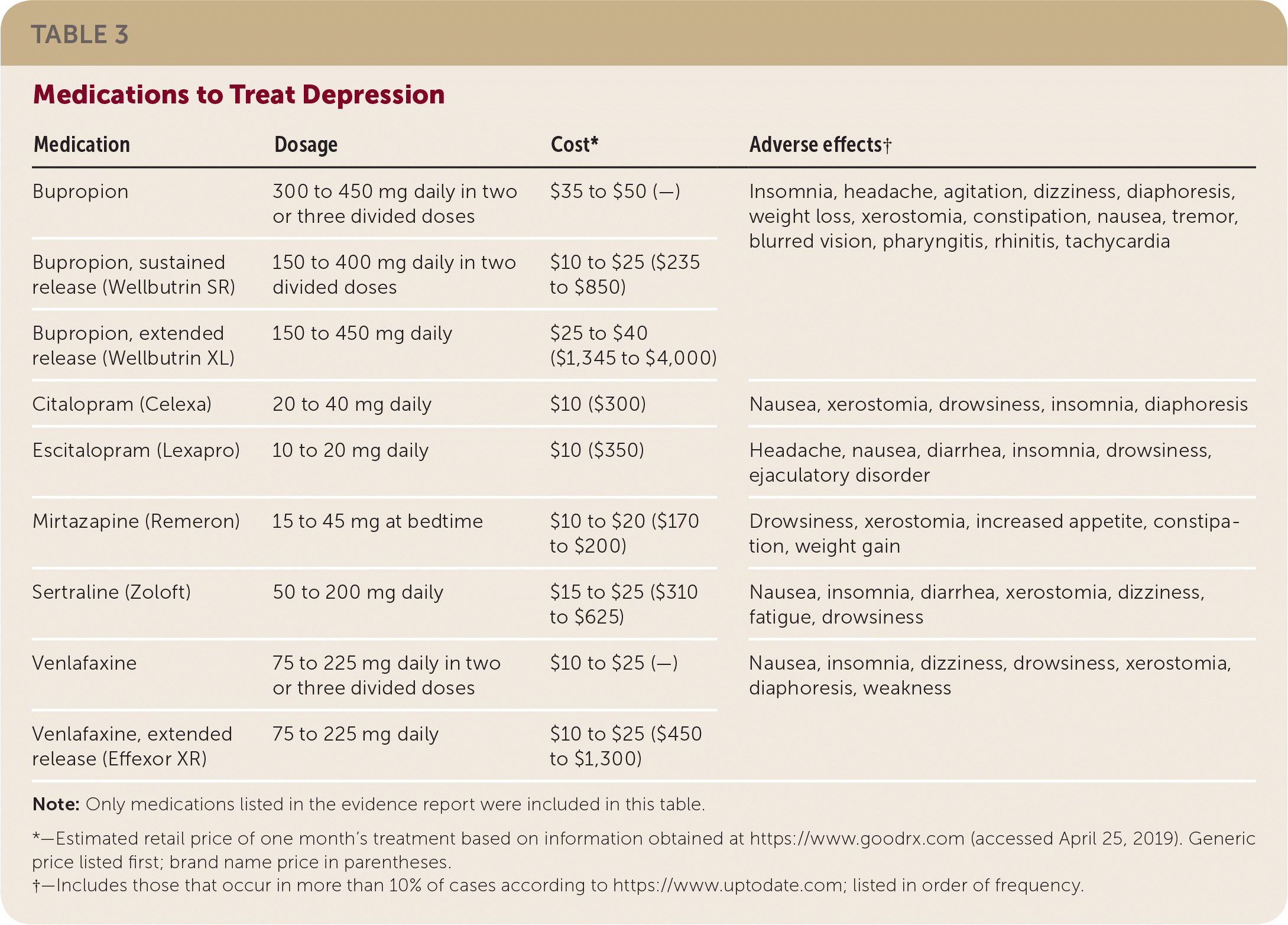
| Medication | Dosage | Cost* | Adverse effects† |
|---|---|---|---|
| Bupropion | 300 to 450 mg daily in two or three divided doses | $35 to $50 (—) | Insomnia, headache, agitation, dizziness, diaphoresis, weight loss, xerostomia, constipation, nausea, tremor, blurred vision, pharyngitis, rhinitis, tachycardia |
| Bupropion, sustained release (Wellbutrin SR) | 150 to 400 mg daily in two divided doses | $10 to $25 ($235 to $850) | |
| Bupropion, extended release (Wellbutrin XL) | 150 to 450 mg daily | $25 to $40 ($1,345 to $4,000) | |
| Citalopram (Celexa) | 20 to 40 mg daily | $10 ($300) | Nausea, xerostomia, drowsiness, insomnia, diaphoresis |
| Escitalopram (Lexapro) | 10 to 20 mg daily | $10 ($350) | Headache, nausea, diarrhea, insomnia, drowsiness, ejaculatory disorder |
| Mirtazapine (Remeron) | 15 to 45 mg at bedtime | $10 to $20 ($170 to $200) | Drowsiness, xerostomia, increased appetite, constipation, weight gain |
| Sertraline (Zoloft) | 50 to 200 mg daily | $15 to $25 ($310 to $625) | Nausea, insomnia, diarrhea, xerostomia, dizziness, fatigue, drowsiness |
| Venlafaxine | 75 to 225 mg daily in two or three divided doses | $10 to $25 (—) | Nausea, insomnia, dizziness, drowsiness, xerostomia, diaphoresis, weakness |
| Venlafaxine, extended release (Effexor XR) | 75 to 225 mg daily | $10 to $25 ($450 to $1,300) |
Four randomized controlled trials evaluated the effectiveness of treating depression in patients following ACS events using pharmacotherapy, CBT, or collaborative care.31–34 Two studies, the COPES and CODIAC trials, compared collaborative care with usual care. The COPES trial included 157 patients with nine months of follow-up, and the CODIAC trial included 150 patients with six months of follow-up.31,32 The patients in the collaborative care group were given the choice of pharmacotherapy, problem-solving therapy (a form of CBT), or both. The patients' symptom severity was evaluated at regular intervals using the BDI, and treatment was adjusted as needed. Usual care involved informing the patients' treating physicians of the diagnosis of depression, who then determined treatment. Both studies found that collaborative care resulted in a significantly greater decrease in symptoms of depression.
The ENRICHD study evaluated CBT and pharmacotherapy compared with usual care in patients within 28 days of an acute myocardial infarction.33 It included 1,784 patients who were followed for 2.4 years. Depression symptoms were assessed using the Hamilton Rating Scale for Depression. The intervention group received CBT and, after five weeks, sertraline was prescribed for those who did not have a greater than 50% reduction in their depression symptoms. Patients in the usual care group received the American Heart Association's Active Partnership Health Education booklet in addition to their primary care clinicians being informed of their diagnosis of depression. The intervention group had a significantly larger decrease in depression symptoms and higher overall life satisfaction scores. However, the difference was no longer significant after 30 months. There was no difference between the intervention and usual care groups in major cardiac events, cardiovascular mortality, cardiovascular hospitalization, or all-cause mortality.
The MIND-IT study compared treatment with antidepressant medication with usual care.34 It included 331 patients and followed their depression symptoms for 18 months and their cardiovascular outcomes for up to five years. Mirtazapine was prescribed as first-line treatment. Citalopram was prescribed to those who did not tolerate or did not respond to mirtazapine after six months. There was no significant difference found in depression symptoms, quality of life, major cardiac events, or cardiac hospitalizations.
The CODIAC study showed greater improvement in symptoms of depression in women than men, whereas the ENRICHD and COPES trials reported no differences between men and women in response to treatment. These studies also reported intervention effects by race and ethnicity and found no statistically significant differences. None of the treatment studies reported adverse events. The U.S. Preventive Services Task Force determined that “second-generation antidepressants (mostly selective serotonin reuptake inhibitors [SSRIs]) are associated with some harms, such as an increase in suicidal behaviors in adults aged 18 to 29 years and an increased risk of upper gastrointestinal bleeding in adults older than 70 years, with risk increasing with age; however, the magnitude of these risks is, on average, small.”35
Although none of these studies demonstrated an effect on cardiovascular mortality or overall mortality, observational studies have suggested that treatment with antidepressant medication, especially SSRIs and SNRIs, may impact these outcomes. The ENRICHD study demonstrated no difference between the intervention and usual care groups, but a post hoc analysis showed a decreased risk of mortality and nonfatal myocardial infarction in patients who took pharmacotherapy, especially SSRIs, independent of what group they were assigned to. Another randomized controlled trial, conducted in Korea, randomized patients to 24 weeks of escitalopram or placebo and showed a statistically significant decrease in myocardial infarction in patients receiving escitalopram after eight years of follow-up. Although not statistically significant, it also demonstrated a trend toward reduced all-cause mortality.36 Although the report on long-term outcomes was published after the completion of the evidence report, the results of the study provide additional support for this recommendation.
Implementations of Recommendations
BARRIERS TO IMPLEMENTATION OF THE GUIDELINE INTO CLINICAL PRACTICE
Studies have identified physician-, practice-, and patient-related factors that may impact the implementation of clinical practice guidelines. Physician-related factors include discomfort with the topic, lack of time, lack of reimbursement, lack of institutional support for screening, and the assumption that the patient would bring up the topic if affected.37–39 Practice-related factors include time constraints on ancillary staff to perform screening, a perceived lack of support from the physician or institution, and the lack of behavioral health staff to assist in the management and counseling of patients identified as having depression. Patient-related factors include the fear of burdening family or friends, the lack of interest in or knowledge about the subject, spiritual or cultural traditions about the topic, and the patient expectation that the physician would initiate the discussion.37–39
Specific to screening for depression, some physicians may have a perceived lack of ability to adequately treat behavioral health issues once identified or limited access to behavioral health services. For the physician working in a rural or underserved region, this lack of behavioral health referral services can be a significant barrier. Patients may feel that they are a family burden and therefore may be concerned about increasing the burden by acknowledging their symptoms of depression. Some individuals may be influenced by spiritual or cultural norms about behavioral health issues leading them to be particularly reticent about the topic.
Patient sex/gender and race/ethnicity should also be considered for implementation of these recommendations. Disparities exist in diagnosis, presentation, treatment, and outcomes for women who present with ACS.40 Many of these differences are because of differences in pathophysiology and mechanisms of heart disease between women and men. However, some disparities may be because of sex-/gender-related differences in the counseling and treatment they receive, including referral to cardiac rehabilitation, an important step in recovery that has been shown to reduce depression post–myocardial infarction. According to a study published in the Journal of the American Heart Association, women are 12% less likely to be referred to cardiac rehabilitation services in comparison with men. Race also plays a role. Compared with white patients, black, Hispanic, and Asian patients are 20%, 36%, and 50%, respectively, less likely to be referred to cardiac rehabilitation.41 In addition, women have been shown to have significantly higher rates of depression than men.9 This is consistent with studies observing higher levels of perceived stress in women and lower quality of life following an ACS event, which was related to a higher rate of comorbidities and higher baseline stress.42,43 Additionally, race and cultural differences can play a role in response to treatment. Shared decision-making is essential in determining treatment plans. Any patient education materials should be written at an appropriate literacy level with risks and benefits of treatment conveyed adequately (Table 3). Psychotherapy and collaborative care interventions that involve family members and attend to cultural values and beliefs may be more successful in some minority populations.
TECHNIQUES FOR IMPLEMENTATION
As described in the preceding sections, there are several general screening tools for depression that can be used for screening of patients who have ACS. The selection of which screening tool to use may be influenced by previous experience with a particular tool, ease of use, accuracy for the given patient population, and reproducibility of meaningful results when administered by the various members of the practice team. Decisions regarding the choice of tool, the timing for screening during a visit, and the team member who provides screening depends on each clinic's preference and workflow. Use of champions at the practice and organizational level can be helpful when implementing a new screening protocol to increase effectiveness and uptake.44 Champions should be comfortable with identification and treatment of this patient population and can subsequently impart the importance of this endeavor to other practice members. Characteristics of these champions can include a willingness to engage others, critical thinking abilities, willingness to adapt and change, commitment to working through setbacks, and willingness to act as ambassadors for the project.45
Lack of access to behavioral health services, in addition to other social determinants of health, which affect patients' ability to obtain services, could be a significant impediment to implementation of these recommendations. Practices should consider screening for social determinants of health and help address those issues by developing and maintaining a directory of resources for patients.46 Increasingly, private and governmental insurers are employing case/care managers to assist patients and providers in obtaining the additional resources needed for supporting patients who face challenging situations. Clinicians may also consider the availability of telemedicine psychological resources, which are becoming increasingly available for rural and underserved populations.
Conclusions and Future Research
The purpose of this updated guideline is to provide clinical recommendations for primary care physicians to appropriately identify and treat patients who have depression following an ACS event. Clinicians should screen patients who have recently experienced an ACS event. When screening, a standardized depression symptom checklist should be used. Patients who have a history of ACS and have been diagnosed with depression should be treated to improve depressive symptoms. Options for treatment of depression in patients who have ACS include antidepressant medication, preferably SSRIs or SNRIs, and/or CBT. Treatment decisions should be based on patient preferences and values and involve shared decision-making by the patient and clinician. This guideline was developed using available evidence; however, significant gaps were identified in the AHRQ systematic review and by members of the guideline development group. New research into these areas may affect the recommendations, at which time the guideline will be updated accordingly. Research that would provide important information for the clinical questions discussed earlier include the following:
Effectiveness data for short screening instruments for depression.
The evidence report found limited data that short screening instruments, item 1 from the BDI-II and the PHQ-2, were as effective as the longer instruments. If these findings were confirmed in larger studies, their use could increase feasibility and implementation of screening for depression in patients who have had a recent ACS event.
Studies examining the effectiveness of screening in subpopulations that may receive disparate treatment (e.g., based on sex, age, ethnicity) and in different settings.
The differences in diagnosis, treatment, and outcomes of women presenting with ACS have been documented.40,47 More data on other populations and in women with depression following ACS events would be valuable for appropriate treatment of these patients. Studies examining the effectiveness of screening for depression immediately after an ACS event in the hospital compared with later screening in the primary care setting would also be beneficial.
Studies examining the effectiveness of different treatments, including the role of dual therapy, the effect of treatment on cardiovascular outcomes, and the benefits and harms of medication with CBT compared with medication alone.
Studies of the effectiveness of exercise, cardiac rehabilitation, and other treatments on depression after ACS events were not identified or did not meet inclusion criteria. There is some evidence that these treatments have a positive effect on depression in addition to effects on cardiovascular outcomes.48 More studies are needed to confirm these findings. Additional long-term and larger studies of antidepressants are needed to confirm effects of these medications on cardiovascular outcomes as indicted in the recent report on a trial with escitalopram in patients following an ACS event.36 Finally, the current evidence supports treatment of patients who have depression following an ACS event with medications and/or CBT. All of the included studies compared treatment with usual care. Direct comparisons of CBT plus medication with medication alone would increase the strength of the evidence and may allow for more informed discussions on treatment options.