Am Fam Physician. 2019;99(12):751-759
Author disclosure: No relevant financial affiliations.
Globally, approximately 20% of the 400 million individuals with diabetes mellitus have diabetic kidney disease (DKD). DKD is associated with higher cardiovascular and all-cause morbidity and mortality, so timely diagnosis and treatment are critical. Screening for early DKD is best done with annual spot urine albumin/creatinine ratio testing, and diagnosis is confirmed by repeated elevation in urinary albumin excretion. Treatment includes management of hyperglycemia, hypertension, hyperlipidemia, and cessation of tobacco use. Multiple antihyperglycemic medications, including sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and dipeptidyl-peptidase-4 inhibitors, may help prevent DKD by lowering blood glucose levels and through intrinsic renal protection. Blood pressure should be monitored at every clinical visit and maintained at less than 140/90 mm Hg to prevent microvascular changes. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers prevent progression of DKD and may decrease albuminuria. Statin therapy should be considered for all patients with DKD, and tobacco cessation reduces the risk of DKD. Given the complexity of the disease and the risk of poor outcomes, patients who progress to stage 3 DKD or beyond may benefit from referral to nephrology subspecialists.
Globally, more than 400 million people have diabetes mellitus and almost 600 million may be affected by 2035.1 In the United States, approximately 12% of the population has diabetes, and up to 25% of these individuals may be undiagnosed.2 The disease affects patients across all age groups, sexes, racial or ethnic groups, education levels, and income levels.2 Diabetic kidney disease (DKD) affects about 20% of patients with diabetes.3 DKD is associated with increased risks of morbidity and mortality and is the leading cause of end-stage renal disease (ESRD) in the United States.4,5
Prevention of diabetes in the general population is the most effective means of minimizing the impact of DKD; understanding risk factors for DKD development can help with early identification and intervention. Effectively using screening guidelines, treatment strategies, and subspecialty referral can help prevent progression of DKD. The role of primary care physicians in the management of patients with DKD secondary to type 2 diabetes is reviewed.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Individuals with type 2 diabetes mellitus should be screened for albuminuria at the time of diagnosis and annually thereafter. | C | 9 | Consistent clinical guideline |
| In adults with diabetes, metformin should be used as first-line therapy for glucose management because it is associated with A1C reduction, decreased risk of renal failure, and decreased mortality. | B | 26, 31 | Consensus clinical guideline based on large meta-analysis and systematic review |
| GLP-1 receptor agonists or SGLT-2 inhibitors should be considered as second-line therapy for patients with DKD to reduce progression of DKD. | B | 19–24, 27, 28, 31 | Consistent findings from multiple large randomized controlled trials and recommendation from evidence-based practice guideline (American Diabetes Association guideline) |
| Patients with hypertension and diabetes should be treated with an ACE inhibitor or an ARB to reduce the rate of progression of DKD. | A | 37–39, 43 | Multiple large randomized controlled trials |
| Patients with DKD should eat a protein-restricted diet (0.8 g per kg per day). | C | 48, 49 | Large meta-analysis |
| For women of reproductive age with diabetes, ACE inhibitor or ARB therapy should be initiated only after discussion of potentially teratogenic effects. | C | 51 | Expert-based clinical guideline |
Pathophysiology
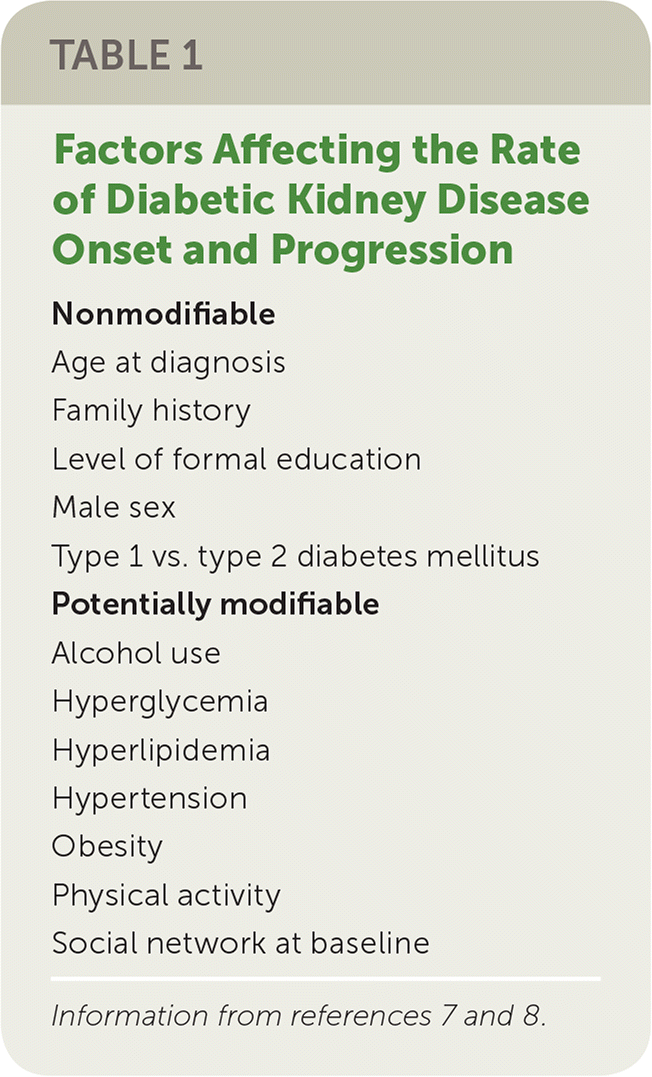
DKD has multiple pathophysiologic mechanisms involving microvascular and macrovascular changes. These changes lead to albuminuria, decreased glomerular filtration, or both. Time to development of DKD varies by pathophysiology of diabetes, age at diagnosis, and a number of other risk factors; its incidence is approximately 2% of patients with diabetes per year6 (Table 17,8). DKD classically progresses from microalbuminuria (30 to 300 mg per day) to macroalbuminuria (more than 300 mg per day) and affects 25% of patients within 10 years of a type 2 diabetes diagnosis.6 These changes may correlate chronologically with the development of diabetic retinopathy.6 Rates of cardiovascular morbidity and mortality rise dramatically with the progression of renal disease. For patients who develop macroalbuminuria, in any given year the risk of mortality (4.6%) is higher than the risk of progression to ESRD (2.3%).6
| Nonmodifiable |
| Age at diagnosis |
| Family history |
| Level of formal education |
| Male sex |
| Type 1 vs. type 2 diabetes mellitus |
| Potentially modifiable |
| Alcohol use |
| Hyperglycemia |
| Hyperlipidemia |
| Hypertension |
| Obesity |
| Physical activity |
| Social network at baseline |
Screening and Diagnosis
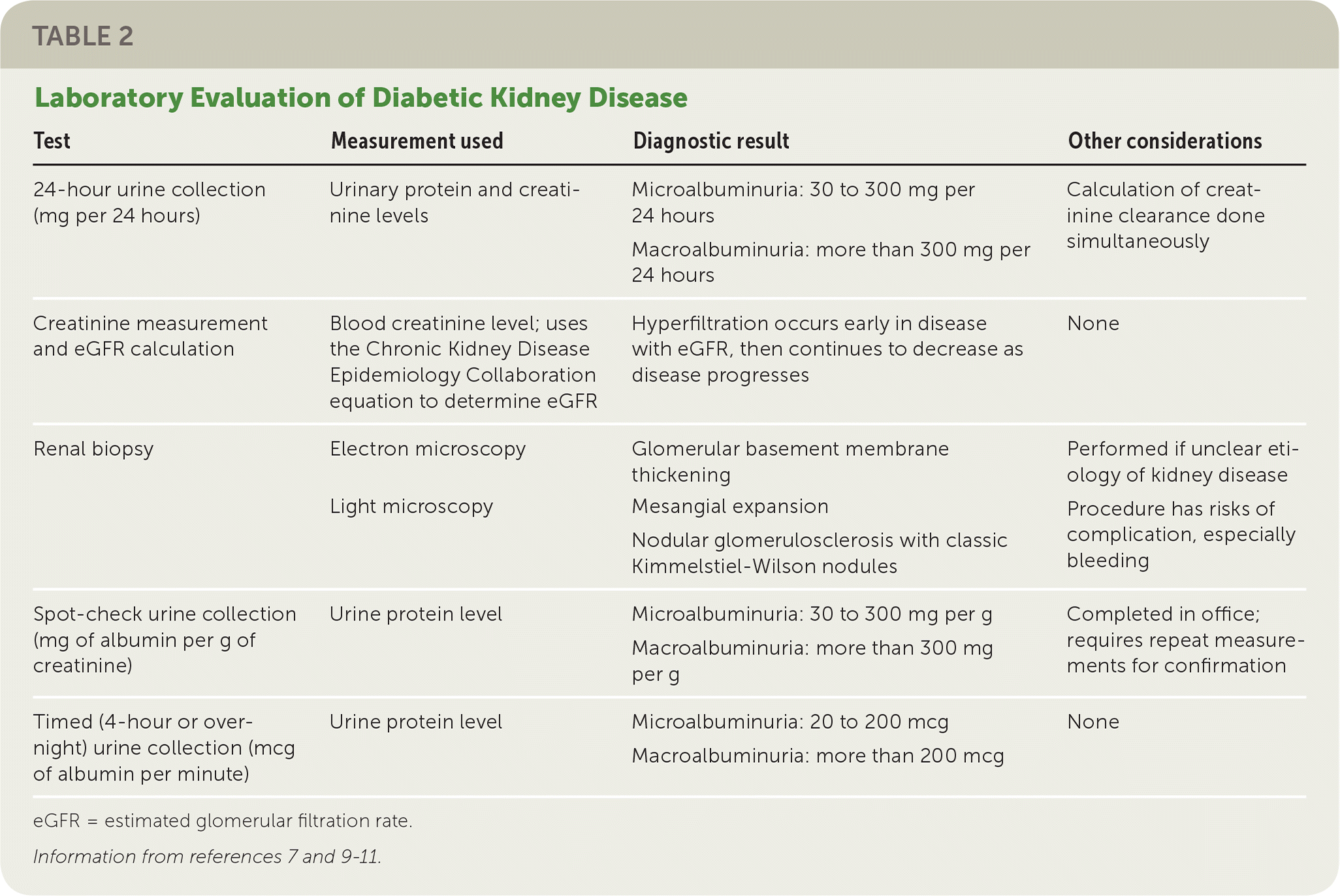
Microalbuminuria is the earliest detectable marker of DKD and is defined as elevated levels of albumin in the urine9 (Table 27,9–11). The American Diabetes Association recommends annual screening of urinary albumin (spot urine albumin/creatinine ratio) and estimated glomerular filtration rate (eGFR) in patients who have had type 1 diabetes for at least five years, in all patients with type 2 diabetes beginning at the time of diagnosis, and in all patients who have comorbid hypertension.9
| Test | Measurement used | Diagnostic result | Other considerations |
|---|---|---|---|
| 24-hour urine collection (mg per 24 hours) | Urinary protein and creatinine levels | Microalbuminuria: 30 to 300 mg per 24 hours Macroalbuminuria: more than 300 mg per 24 hours | Calculation of creatinine clearance done simultaneously |
| Creatinine measurement and eGFR calculation | Blood creatinine level; uses the Chronic Kidney Disease Epidemiology Collaboration equation to determine eGFR | Hyperfiltration occurs early in disease with eGFR, then continues to decrease as disease progresses | None |
| Renal biopsy | Electron microscopy Light microscopy | Glomerular basement membrane thickening Mesangial expansion Nodular glomerulosclerosis with classic Kimmelstiel-Wilson nodules | Performed if unclear etiology of kidney disease Procedure has risks of complication, especially bleeding |
| Spot-check urine collection (mg of albumin per g of creatinine) | Urine protein level | Microalbuminuria: 30 to 300 mg per g Macroalbuminuria: more than 300 mg per g | Completed in office; requires repeat measurements for confirmation |
| Timed (4-hour or over-night) urine collection (mcg of albumin per minute) | Urine protein level | Microalbuminuria: 20 to 200 mcg Macroalbuminuria: more than 200 mcg | None |
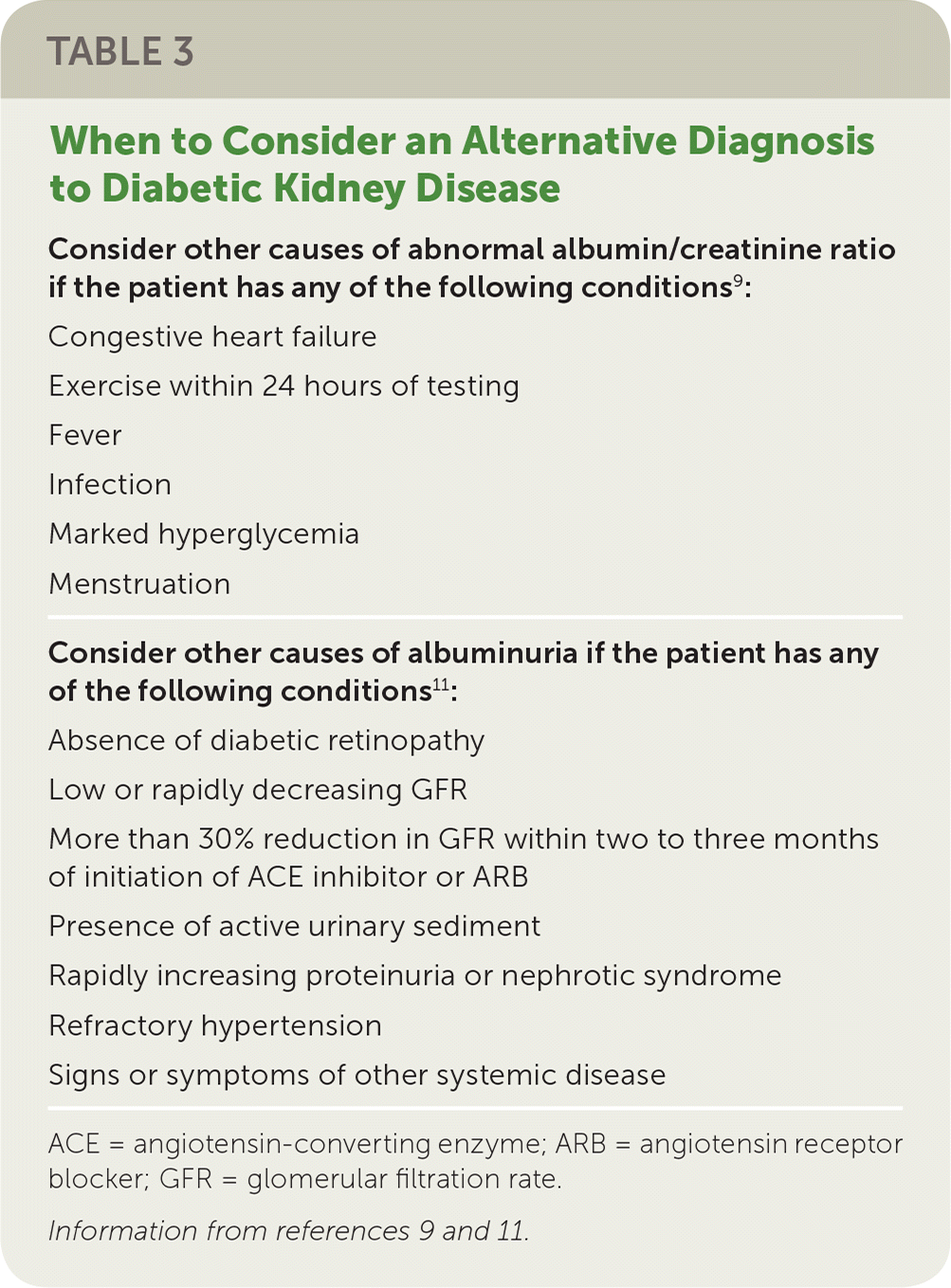
Screening for microalbuminuria can be completed in three ways: 24-hour collection with creatinine, allowing for simultaneous measurement of creatinine clearance; timed (four-hour or overnight) collection; or measurement of the albumin/creatinine ratio in a random spot collection.9 The spot collection can easily be completed in the office and is usually the preferred method.9 Because the albumin/creatinine ratio can be elevated independent of kidney damage, consideration should be given to the possibility of other causes of an elevated urine albumin/creatinine ratio (Table 3).9,11 Because of this variability in urinary albumin excretion, two of three urine albumin/creatinine ratio specimens collected over a three- to six-month period must be abnormal (30 mg albumin per g creatinine to 300 mg albumin per g creatinine) before diagnosis of microalbuminuria can be made.9 Macroalbuminuria (more than 300 mg per g) on a single sample can confirm diagnosis in the absence of complicating factors11 (Table 39,11). Patients are more likely to develop ESRD if they have persistent and severely increased levels of albuminuria (300 mg per g or higher).
| Consider other causes of abnormal albumin/creatinine ratio if the patient has any of the following conditions9: |
| Congestive heart failure |
| Exercise within 24 hours of testing |
| Fever |
| Infection |
| Marked hyperglycemia |
| Menstruation |
| Consider other causes of albuminuria if the patient has any of the following conditions11: |
| Absence of diabetic retinopathy |
| Low or rapidly decreasing GFR |
| More than 30% reduction in GFR within two to three months of initiation of ACE inhibitor or ARB |
| Presence of active urinary sediment |
| Rapidly increasing proteinuria or nephrotic syndrome |
| Refractory hypertension |
| Signs or symptoms of other systemic disease |
Screening for DKD should also include measurement of serum creatinine and eGFR.11 The eGFR should be calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation12 (https://www.mdcalc.com/ckd-epi-equations-glomerular-filtration-rate-gfr).
Diagnosis is made clinically when a patient has evidence of kidney disease and no other primary etiology. Early referral to nephrology (at chronic kidney disease stage 3 or 4) may help improve DKD outcomes and should be considered.
Treatment
Identification of patients with microalbuminuria allows for timely initiation of treatment to prevent disease progression and to reduce the risk of ESRD. Treatment of DKD primarily involves careful management of hyperglycemia and hypertension with use of medications that confer specific renal benefit. Attention should also be paid to other potentially modifiable risk factors (Table 17,8).
GLYCEMIC CONTROL
No large trials have specifically evaluated ideal glycemic targets to prevent DKD, but multiple studies have sought to clarify the optimal level of glycemic control to prevent macrovascular (e.g., myocardial infarction, stroke, death) and microvascular (e.g., retinopathy, DKD) complications of diabetes.13–15 The American Diabetes Association recommends a target A1C level less than 7% for many adults, whereas the 2018 guideline from the American College of Physicians suggests that a target of 7% to 8% may be more appropriate.16,17 A lower A1C target (e.g., less than 6% vs. 7% to 8%) has been associated with a reduction in DKD but at the cost of more hypoglycemic events, polypharmacy, and increased mortality.14,18 Variations in target A1C levels may be appropriate for some patients (Table 4).17,18
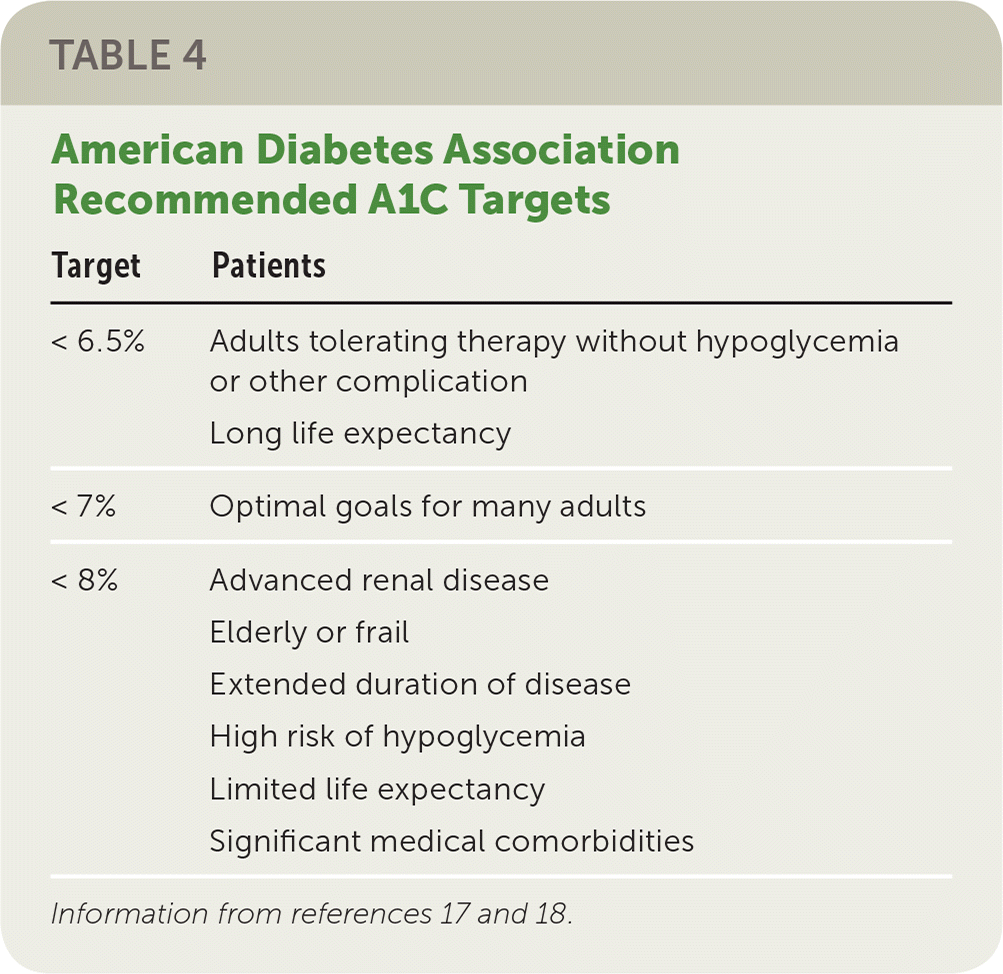
| Target | Patients |
|---|---|
| < 6.5% | Adults tolerating therapy without hypoglycemia or other complication Long life expectancy |
| < 7% | Optimal goals for many adults |
| < 8% | Advanced renal disease Elderly or frail Extended duration of disease High risk of hypoglycemia Limited life expectancy Significant medical comorbidities |
A1C measurements in patients with chronic kidney disease stage 4 or 5 may be falsely low because of shortened red-cell survival time and associated chronic anemia. In these circumstances, routine glucose monitoring may be more accurate for testing and treatment planning.
Hyperglycemia should be managed with a multifactorial approach, including weight loss, exercise, diet modification, and medication. Lifestyle changes and metformin remain the first-line therapy for patients with diabetes. Secondary data analyses of intermediate renal outcomes in large trials suggest that medications from multiple drug classes may help reduce progression to DKD independent of their glucose-lowering mechanisms (Table 5).19–29 Notably, glucagon-like peptide-1 receptor agonists and dipeptidyl-peptidase-4 inhibitors reduce progression of albuminuria, and sodium-glucose cotransporter-2 inhibitors decrease rates of progression of renal disease and need for renal replacement therapy.20,22,24,27,28,30 Because of this intrinsic renal protection, glucagon-like peptide-1 agonists and sodium-glucose cotransporter-2 inhibitors are recommended as second-line therapy for patients who do not achieve their target A1C with lifestyle changes and metformin alone.31
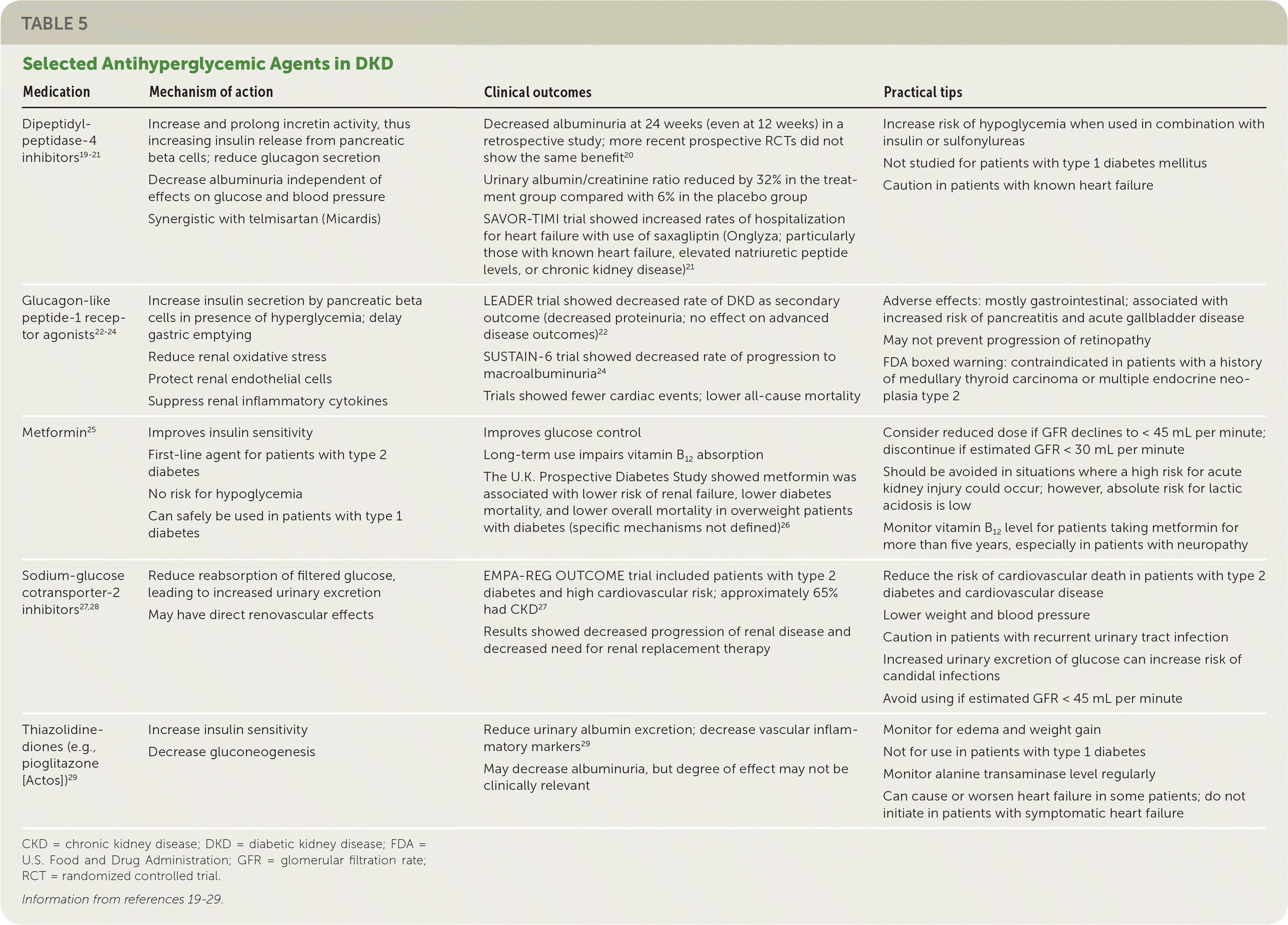
| Medication | Mechanism of action | Clinical outcomes | Practical tips |
|---|---|---|---|
| Dipeptidyl-peptidase-4 inhibitors19–21 | Increase and prolong incretin activity, thus increasing insulin release from pancreatic beta cells; reduce glucagon secretion Decrease albuminuria independent of effects on glucose and blood pressure Synergistic with telmisartan (Micardis) | Decreased albuminuria at 24 weeks (even at 12 weeks) in a retrospective study; more recent prospective RCTs did not show the same benefit20 Urinary albumin/creatinine ratio reduced by 32% in the treatment group compared with 6% in the placebo group SAVOR-TIMI trial showed increased rates of hospitalization for heart failure with use of saxagliptin (Onglyza; particularly those with known heart failure, elevated natriuretic peptide levels, or chronic kidney disease)21 | Increase risk of hypoglycemia when used in combination with insulin or sulfonylureas Not studied for patients with type 1 diabetes mellitus Caution in patients with known heart failure |
| Glucagon-like peptide-1 receptor agonists22–24 | Increase insulin secretion by pancreatic beta cells in presence of hyperglycemia; delay gastric emptying Reduce renal oxidative stress Protect renal endothelial cells Suppress renal inflammatory cytokines | LEADER trial showed decreased rate of DKD as secondary outcome (decreased proteinuria; no effect on advanced disease outcomes)22 SUSTAIN-6 trial showed decreased rate of progression to macroalbuminuria24 Trials showed fewer cardiac events; lower all-cause mortality | Adverse effects: mostly gastrointestinal; associated with increased risk of pancreatitis and acute gallbladder disease May not prevent progression of retinopathy FDA boxed warning: contraindicated in patients with a history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 |
| Metformin25 | Improves insulin sensitivity First-line agent for patients with type 2 diabetes No risk for hypoglycemia Can safely be used in patients with type 1 diabetes | Improves glucose control Long-term use impairs vitamin B12 absorption The U.K. Prospective Diabetes Study showed metformin was associated with lower risk of renal failure, lower diabetes mortality, and lower overall mortality in overweight patients with diabetes (specific mechanisms not defined)26 | Consider reduced dose if GFR declines to < 45 mL per minute; discontinue if estimated GFR < 30 mL per minute Should be avoided in situations where a high risk for acute kidney injury could occur; however, absolute risk for lactic acidosis is low Monitor vitamin B12 level for patients taking metformin for more than five years, especially in patients with neuropathy |
| Sodium-glucose cotransporter-2 inhibitors27,28 | Reduce reabsorption of filtered glucose, leading to increased urinary excretion May have direct renovascular effects | EMPA-REG OUTCOME trial included patients with type 2 diabetes and high cardiovascular risk; approximately 65% had CKD27 Results showed decreased progression of renal disease and decreased need for renal replacement therapy | Reduce the risk of cardiovascular death in patients with type 2 diabetes and cardiovascular disease Lower weight and blood pressure Caution in patients with recurrent urinary tract infection Increased urinary excretion of glucose can increase risk of candidal infections Avoid using if estimated GFR < 45 mL per minute |
| Thiazolidine-diones (e.g., pioglitazone [Actos])29 | Increase insulin sensitivity Decrease gluconeogenesis | Reduce urinary albumin excretion; decrease vascular inflammatory markers29 May decrease albuminuria, but degree of effect may not be clinically relevant | Monitor for edema and weight gain Not for use in patients with type 1 diabetes Monitor alanine transaminase level regularly Can cause or worsen heart failure in some patients; do not initiate in patients with symptomatic heart failure |
BLOOD PRESSURE CONTROL
Blood pressure (BP) control is critical to prevent and slow the progression of DKD. BP should be monitored at every routine clinical visit.32 Standard BP monitoring and diagnosis guidelines should be applied (including having the patient seated with feet on the floor, arm supported, and the use of ambulatory BP monitoring). There is some variation in guideline recommendations for target BP for patients with diabetes and DKD (Table 632–34). Several large studies have attempted to identify the safest BP thresholds, but differences in study design, enrollment criteria, and treatment duration complicate development of a clear, single goal.34–36
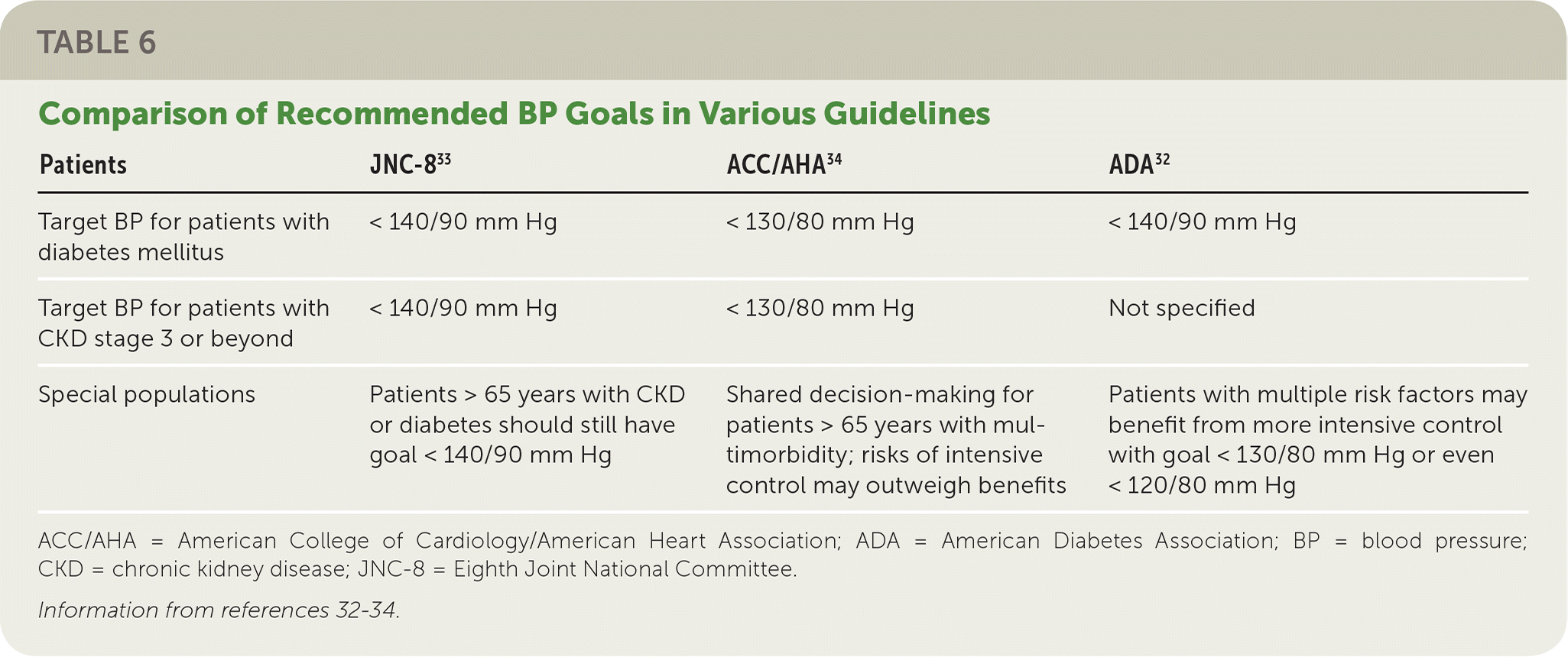
| Patients | JNC-833 | ACC/AHA34 | ADA32 |
|---|---|---|---|
| Target BP for patients with diabetes mellitus | < 140/90 mm Hg | < 130/80 mm Hg | < 140/90 mm Hg |
| Target BP for patients with CKD stage 3 or beyond | < 140/90 mm Hg | < 130/80 mm Hg | Not specified |
| Special populations | Patients > 65 years with CKD or diabetes should still have goal < 140/90 mm Hg | Shared decision-making for patients > 65 years with multimorbidity; risks of intensive control may outweigh benefits | Patients with multiple risk factors may benefit from more intensive control with goal < 130/80 mm Hg or even < 120/80 mm Hg |
To reduce rates of microvascular disease (including DKD), systolic BP should be maintained at less than 140 mm Hg, and diastolic BP should be maintained at less than 90 mm Hg.35 Lower targets (130/80 mm Hg) may be appropriate for some patients (e.g., those with known DKD or other increased risk of atherosclerotic cardiovascular disease) if they can be achieved without significant treatment burden or adverse effects.
One large trial of patients with diabetes found no significant difference in adverse cardiovascular outcomes between standard control (BP less than 140/90 mm Hg) and intensive control (target BP less than 120/80 mm Hg; P = .20). Higher rates of adverse outcomes in the intensive therapy arm, including significant reductions in eGFR and increases in macroalbuminuria (number needed to harm = 47), suggest that the risks of aggressive BP control may outweigh any benefits.36
Initial treatment of hypertension in patients with diabetes should involve lifestyle management. This includes dietary sodium restriction (less than 2,300 mg per day), weight loss if overweight or obese, increased physical activity, and moderation of alcohol intake.32 A patient-specific treatment plan should be discussed at the time of diagnosis and accompanied by pharmacologic therapy to achieve target BP (Table 737–43).
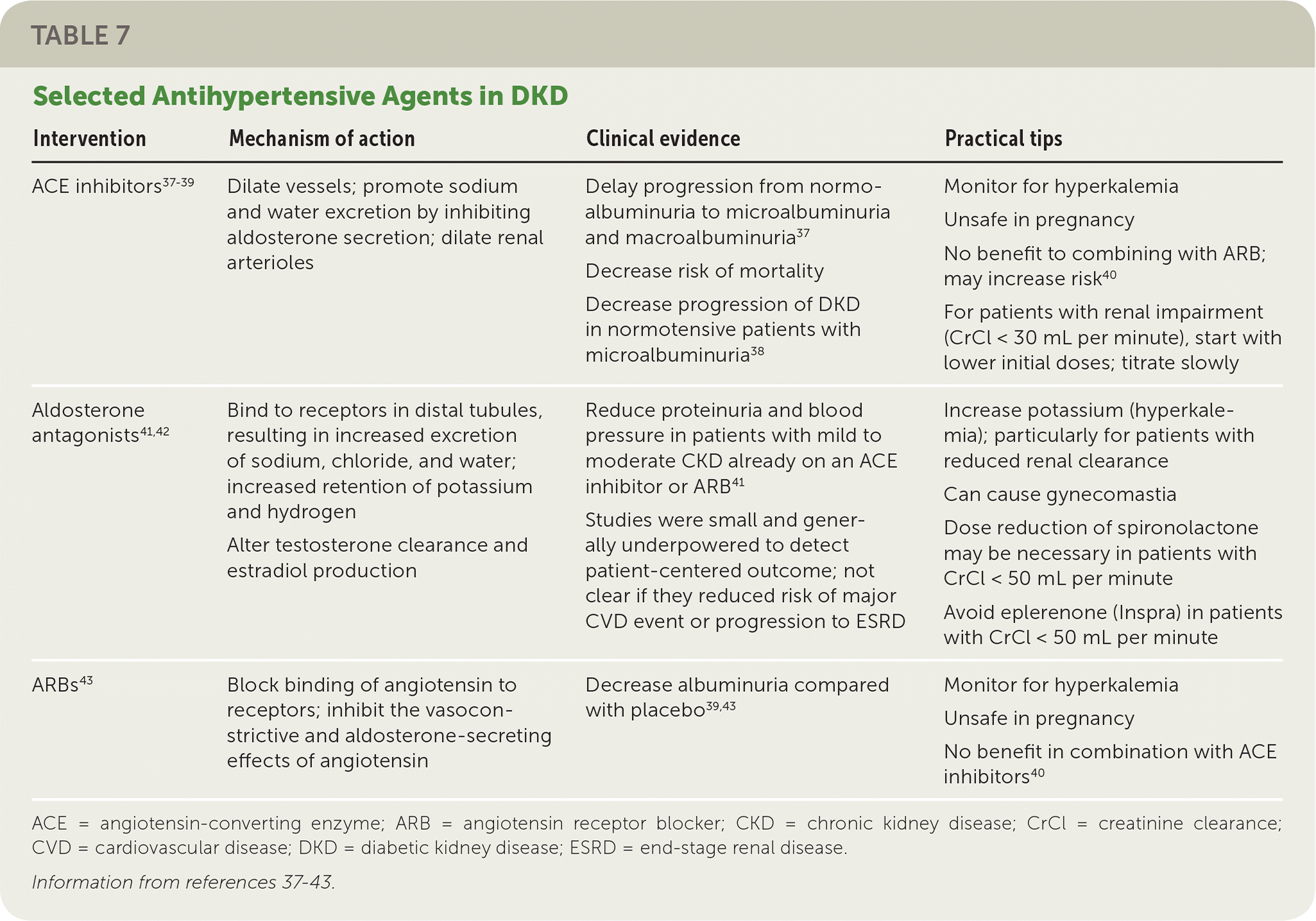
| Intervention | Mechanism of action | Clinical evidence | Practical tips |
|---|---|---|---|
| ACE inhibitors37–39 | Dilate vessels; promote sodium and water excretion by inhibiting aldosterone secretion; dilate renal arterioles | Delay progression from normoalbuminuria to microalbuminuria and macroalbuminuria37 Decrease risk of mortality Decrease progression of DKD in normotensive patients with microalbuminuria38 | Monitor for hyperkalemia Unsafe in pregnancy No benefit to combining with ARB; may increase risk40 For patients with renal impairment (CrCl < 30 mL per minute), start with lower initial doses; titrate slowly |
| Aldosterone antagonists41,4 | Bind to receptors in distal tubules, resulting in increased excretion of sodium, chloride, and water; increased retention of potassium and hydrogen Alter testosterone clearance and estradiol production | Reduce proteinuria and blood pressure in patients with mild to moderate CKD already on an ACE inhibitor or ARB41 Studies were small and generally underpowered to detect patient-centered outcome; not clear if they reduced risk of major CVD event or progression to ESRD | Increase potassium (hyperkalemia); particularly for patients with reduced renal clearance Can cause gynecomastia Dose reduction of spironolactone may be necessary in patients with CrCl < 50 mL per minute Avoid eplerenone (Inspra) in patients with CrCl < 50 mL per minute |
| ARBs43 | Block binding of angiotensin to receptors; inhibit the vasoconstrictive and aldosterone-secreting effects of angiotensin | Decrease albuminuria compared with placebo39,43 | Monitor for hyperkalemia Unsafe in pregnancy No benefit in combination with ACE inhibitors40 |
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) delay and reduce the progression of DKD.37–39,43 A 2012 Cochrane review concluded that ACE inhibitors reduce the risk of new onset microalbuminuria or macroalbuminuria in individuals with diabetes with or without hypertension.37 The same review found that ACE inhibitors reduce the risk of death in patients with diabetes compared with placebo.37 In 2011, a large randomized controlled trial showed that olmesartan (Benicar) delays the onset of microalbuminuria compared with placebo (even though both groups achieved BP targets); a 2014 follow-up showed that this benefit is sustained over time.39,43 No added benefit to dual therapy with ACE inhibitors and ARBs occurs, whereas the risks of hyperkalemia, hypotension, and renal failure increase.44
Aldosterone antagonists have therapeutic benefit in combination with ACE inhibitors or ARBs, but the risk of hyperkalemia is high; therefore, they must be prescribed with careful monitoring.41 Calcium channel blockers and thiazide diuretics have been shown to exhibit cardioprotection, but they do not appear to have the same degree of benefit on preventing progression of DKD.45
LIPID MANAGEMENT
DKD alters lipid metabolism, leading to increased low-density lipoprotein–cholesterol complex and increasing risk of poor outcomes attributable to atherosclerotic cardiovascular disease. Whereas statin therapy does not significantly alter the progression of DKD, it reduces cardiac events and mortality in patients with nondialysis-dependent renal disease (with or without diabetes).5 Many statins are metabolized by the kidneys; therefore, doses should be reduced if a patient has significantly decreased eGFR. Atorvastatin (Lipitor) doses do not need to be adjusted.
Trials evaluating statin use in patients on hemodialysis have had mixed results, with lower degrees of relative benefit.46,47 Discussion and shared decision-making about the initiation and continuation of statin therapy are appropriate for patients with diabetes of all ages and at all stages of DKD.32
DIETARY MODIFICATION
Dietary modification has the potential for preventing progression of DKD; however, the evidence for specific interventions is mixed. The American Diabetes Association recommends a protein-restricted diet (0.8 g per kg per day) in patients with DKD, based on studies that show that this can slow the decline of GFR and progression to ESRD.48,49 A Mediterranean diet and the dietary approaches to stop hypertension (DASH) diet can have beneficial outcomes. These diets include whole-grain carbohydrates, fiber, fresh fruits and vegetables, omega-3 and omega-9 fats, and less than 2,300 mg per day of sodium. Foods that are high in sugar, saturated fats, and processed carbohydrates should be avoided.49 In patients who have DKD, routine monitoring for alterations in phosphorus, potassium, and vitamin D may guide additional dietary modification.
CONSIDERATIONS IN CHILDREN AND ADOLESCENTS
The evaluation and treatment of DKD in children and adolescents with types 1 and 2 diabetes are guided by limited evidence. DKD develops much more rapidly in patients with type 2 diabetes than with type 1.50 For this reason, screening should begin within five years of diagnosis of type 1 diabetes (or at age 10 or onset of puberty, whichever comes first) and at the time of diagnosis for patients with type 2 diabetes.50
ACE inhibitor or ARB treatment should be considered for adolescents with elevations in albumin/creatinine ratio and hypertension only after appropriate reproductive counseling for young women about potentially teratogenic effects; ACE inhibitor or ARB treatment should be avoided in women considering pregnancy.51 Because tobacco use increases progression of DKD, adolescents should be counseled to avoid smoking (cigarettes, e-cigarettes).51
Endocrinology and nephrology consultation should be considered early to help with disease management and prevention of complications in younger patients with DKD.
PREGNANCY CONSIDERATIONS
Reproductive education and preconception counseling are critical for all women of childbearing age who have diabetes, but limited data guide management of DKD specifically. Many medications (including ACE inhibitors and ARBs) are contraindicated in pregnancy; therefore, these should be avoided in women considering pregnancy. For women who have diabetes and conceive, recommended glycemic targets (A1C target less than 6% if possible and less than 7% if hypoglycemia occurs52) are different from those for nonpregnant women to support healthy pregnancy and fetal development.
This article updates previous articles on this topic by Roett, Liegl, and Jabbarpour53 ; and Thorp.54
Data Sources: A PubMed search was completed in Clinical Queries using the key term diabetic kidney disease, in combination with the terms diagnosis, treatment, and prevention. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews, with particular attention to recently published manuscripts. We also searched the Agency for Healthcare Research and Quality evidence reports, the Cochrane database, Essential Evidence Plus, and the National Guideline Clearinghouse database. Search dates: May 16, 2018, and February 15, 2019.