Am Fam Physician. 2019;100(2):116-117
Author disclosure: No relevant financial affiliations.
Semaglutide (Ozempic) is a glucagon-like peptide-1 (GLP-1) receptor agonist labeled for the treatment of type 2 diabetes mellitus in adults.1
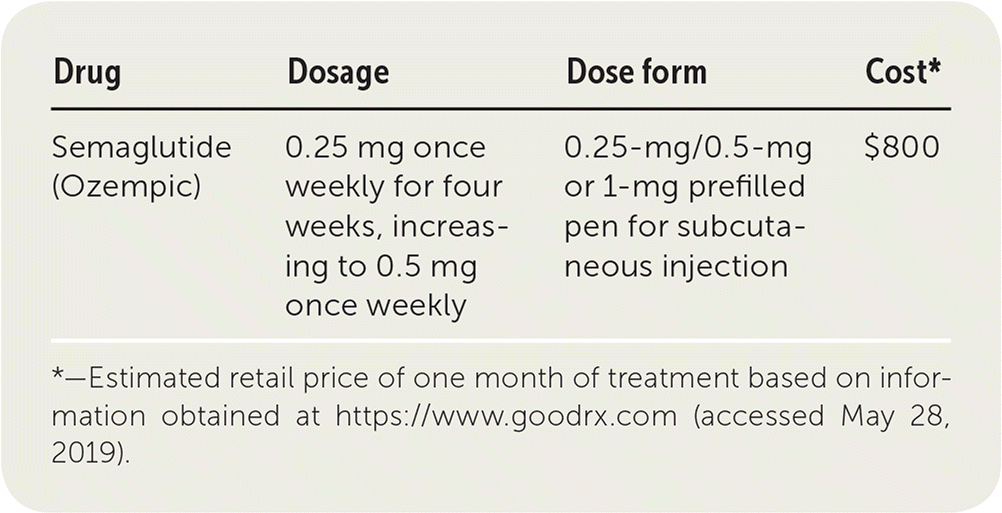
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Semaglutide (Ozempic) | 0.25 mg once weekly for four weeks, increasing to 0.5 mg once weekly | 0.25-mg/0.5-mg or 1-mg prefilled pen for subcutaneous injection | $800 |
Safety
As with other GLP-1 receptor agonists, semaglutide should not be used as first-line treatment because of a potential risk of thyroid cancer, based on animal studies.1 It should not be used in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia, type 2.
When used alone, semaglutide has not been shown to increase the risk of severe hypoglycemia (i.e., glucose levels of 56 mg per dL [3.1 mmol per L] or less) compared with placebo.1 However, the risk is increased when semaglutide is added to other treatments such as basal insulin or a sulfonylurea. The effect of semaglutide on hypoglycemia rates has not been studied when used with metformin alone.
Pancreatitis will occur about three times per 1,000 patient-years. Semaglutide should not be used in patients with a history of pancreatitis because it has not been studied in this patient population.1 Cholelithiasis will affect about 2% of patients per year.1 Diabetic retinopathy complications requiring treatment may be more common with semaglutide than with placebo. In a trial of 3,297 patients, most of whom had preexisting retinopathy, diabetic retinopathy complications occurred in 3% of those taking semaglutide vs. 1.8% of the placebo group.2 This increase has not been documented with other GLP-1 receptor agonists. Patients with a history of diabetic retinopathy should be monitored for disease progression.1
Semaglutide should not be used in children. It has not been studied in pregnant or breastfeeding women, and the manufacturer recommends stopping treatment at least two months before attempting pregnancy.1
Tolerability
Semaglutide is generally well tolerated with similar dropout rates when compared with placebo, although it may be less well tolerated than dulaglutide (Trulicity).1,3 The most common adverse effects are nausea, vomiting, and diarrhea and will be experienced by about 30% of patients, usually when the dose is increased. Approximately 3% to 4% of patients will discontinue treatment because of gastrointestinal adverse effects.1
Effectiveness
Semaglutide has been evaluated as a single treatment and as additional treatment for patients already being treated with metformin, a sulfonylurea, or long-acting insulin in seven clinical trials enrolling more than 8,400 patients. When added to metformin, semaglutide will reduce A1C levels by 1.5 to 1.8 percentage points, depending on the dose. This reduction is slightly larger than when dulaglutide is added.3 Comparable results will be achieved when semaglutide is added to other treatments.1
Patients taking semaglutide will lose an average of 3.8 to 4.7 kg (8.4 to 10.4 lb). Weight loss is greater when patients are also using metformin or insulin. Although it has not been compared directly, this weight loss is greater than with other treatments.1
In a study of 3,297 patients with established cardiovascular disease already on standard treatment, the addition of semaglutide reduced the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke by 2.3 percentage points as compared with placebo (6.6% vs. 8.9%; number needed to treat = 44 over 2.1 years).2 Although not directly compared with semaglutide, liraglutide (Victoza), linagliptin (Tradjenta), and empagliflozin (Jardiance) may also reduce outcomes in patients at high cardiovascular risk.4–6
Price
A one-month supply of semaglutide costs approximately $800. Although it is similar in price to other GLP-1 receptor agonists, it is substantially more expensive than many other glucose-lowering agents.
Simplicity
Semaglutide is a once-weekly subcutaneous injection available as a prefilled pen. It should be stored in a refrigerator until first use but then may be kept at room temperature for up to 56 days. The recommended starting dosage is 0.25 mg once weekly for four weeks, then increased to 0.5 mg once weekly. If additional glycemic control is needed, the dosage should be increased to 1.0 mg once weekly.
Bottom Line
Semaglutide can be added to existing treatment to improve glycemic control and induce weight loss. It may also protect against cardiovascular outcomes in patients with type 2 diabetes and established cardiovascular disease. Some patients may find the once-weekly dosing to be appealing. However, gastrointestinal adverse effects may limit its use for some patients. Given the price, potential harms, and tolerability of semaglutide, other less expensive treatments should be considered first for most patients.